Abstract
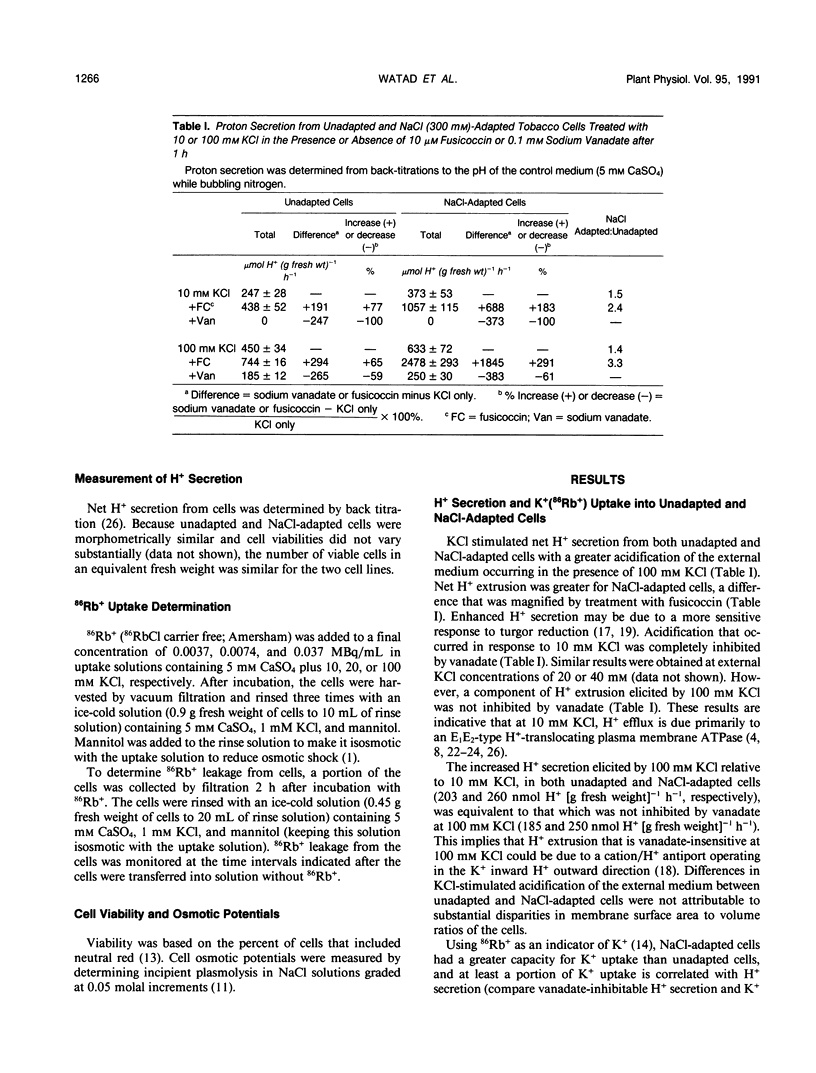
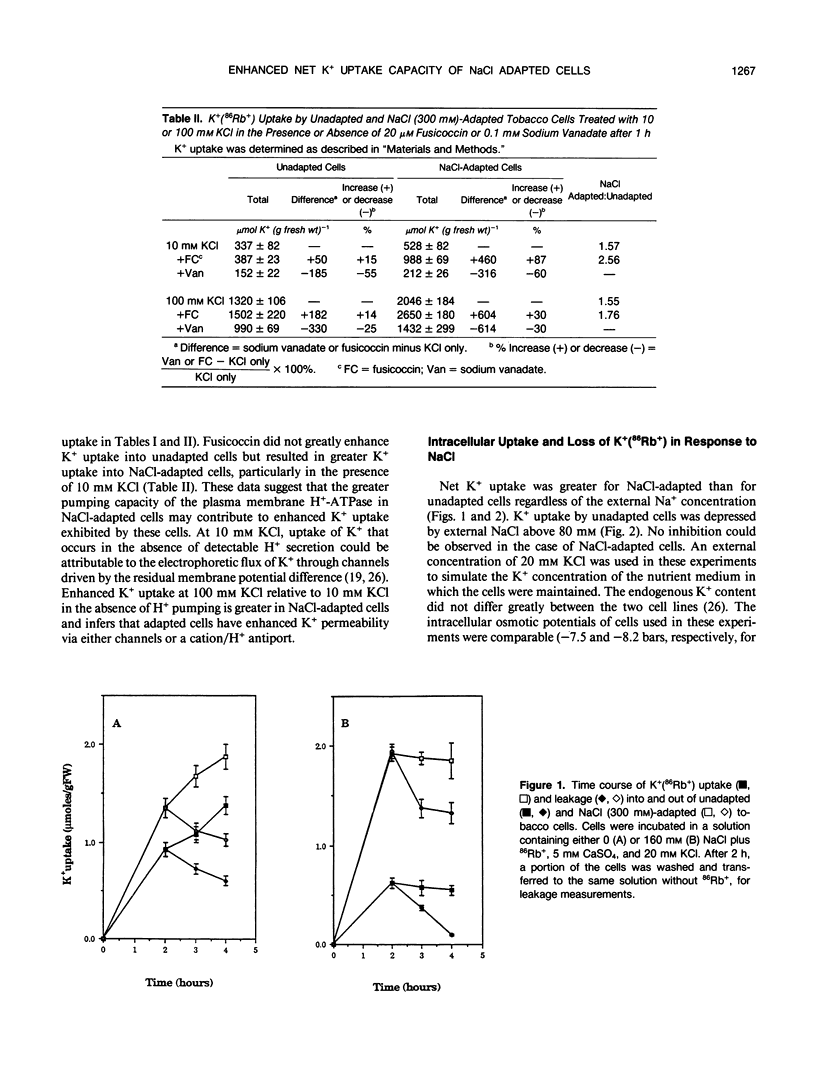
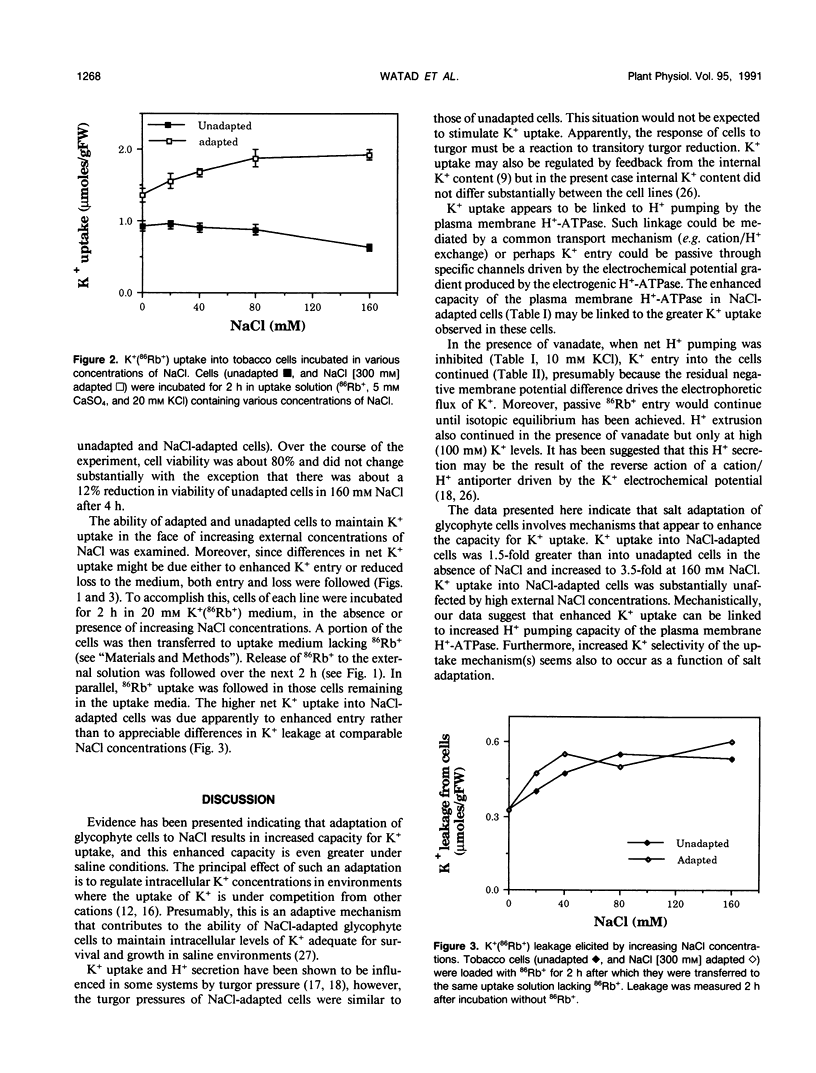
Maintenance of intracellular K+ concentrations that are not growth-limiting, in an environment of high Na+, is characteristic of NaCl-adapted cells of the glycophyte, tobacco (Nicotiana tabacum/gossii). These cells exhibited a substantially greater uptake of 86Rb+ (i.e. an indicator of K+) relative to unadapted cells. Potassium uptake into NaCl-adapted cells was 1.5-fold greater than unadapted cells at 0 NaCl and 3.5-fold greater when cells were exposed to 160 millimolar NaCl. The difference in net K+ uptake between unadapted and NaCl-adapted cells was due primarily to higher rates of entry rather than to reduced K+ leakage. Presumably, enhanced K+ uptake into adapted cells is a result of electrophoretic flux, and a component of uptake may be linked to vanadate-sensitive H+ extrusion.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amar L., Reinhold L. Loss of membrane transport ability in leaf cells and release of protein as a result of osmotic shock. Plant Physiol. 1973 Apr;51(4):620–625. doi: 10.1104/pp.51.4.620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basso B., Ullrich-Eberius C. I. Membrane Potential and Proton Cotransport of Alanine and Phosphate as Affected by Permeant Weak Acids in Lemna gibba. Plant Physiol. 1987 Nov;85(3):674–678. doi: 10.1104/pp.85.3.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binzel M. L., Hess F. D., Bressan R. A., Hasegawa P. M. Intracellular compartmentation of ions in salt adapted tobacco cells. Plant Physiol. 1988 Feb;86(2):607–614. doi: 10.1104/pp.86.2.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun Y., Hassidim M., Lerner H. R., Reinhold L. Studies on H-Translocating ATPases in Plants of Varying Resistance to Salinity : I. Salinity during Growth Modulates the Proton Pump in the Halophyte Atriplex nummularia. Plant Physiol. 1986 Aug;81(4):1050–1056. doi: 10.1104/pp.81.4.1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer G. R., Läuchli A., Polito V. S. Displacement of ca by na from the plasmalemma of root cells : a primary response to salt stress? Plant Physiol. 1985 Sep;79(1):207–211. doi: 10.1104/pp.79.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein E., Rains D. W., Elzam O. E. RESOLUTION OF DUAL MECHANISMS OF POTASSIUM ABSORPTION BY BARLEY ROOTS. Proc Natl Acad Sci U S A. 1963 May;49(5):684–692. doi: 10.1073/pnas.49.5.684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher S. R., Leonard R. T. Effect of vanadate, molybdate, and azide on membrane-associated ATPase and soluble phosphatase activities of corn roots. Plant Physiol. 1982 Nov;70(5):1335–1340. doi: 10.1104/pp.70.5.1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handa A. K., Bressan R. A., Handa S., Hasegawa P. M. Characteristics of cultured tomato cells after prolonged exposure to medium containing polyethylene glycol. Plant Physiol. 1982 Feb;69(2):514–521. doi: 10.1104/pp.69.2.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larosa P. C., Hasegawa P. M., Rhodes D., Clithero J. M., Watad A. E., Bressan R. A. Abscisic Acid Stimulated Osmotic Adjustment and Its Involvement in Adaptation of Tobacco Cells to NaCl. Plant Physiol. 1987 Sep;85(1):174–181. doi: 10.1104/pp.85.1.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Läuchli A., Epstein E. Transport of potassium and rubidium in plant roots: the significance of calcium. Plant Physiol. 1970 May;45(5):639–641. doi: 10.1104/pp.45.5.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhold L., Seiden A., Volokita M. Is modulation of the rate of proton pumping a key event in osmoregulation? Plant Physiol. 1984 Jul;75(3):846–849. doi: 10.1104/pp.75.3.846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuveni M., Colombo R., Lerner H. R., Pradet A., Poljakoff-Mayber A. Osmotically induced proton extrusion from carrot cells in suspension culture. Plant Physiol. 1987 Oct;85(2):383–388. doi: 10.1104/pp.85.2.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romani G., Marrè M. T., Bellando M., Alloatti G., Marrè E. H extrusion and potassium uptake associated with potential hyperpolarization in maize and wheat root segments treated with permeant weak acids. Plant Physiol. 1985 Nov;79(3):734–739. doi: 10.1104/pp.79.3.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schröppel-Meier G., Kaiser W. M. Ion Homeostasis in Chloroplasts under Salinity and Mineral Deficiency : I. Solute Concentrations in Leaves and Chloroplasts from Spinach Plants under NaCl or NaNO(3) Salinity. Plant Physiol. 1988 Aug;87(4):822–827. doi: 10.1104/pp.87.4.822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schröppel-Meier G., Kaiser W. M. Ion Homeostasis in Chloroplasts under Salinity and Mineral Deficiency: II. Solute Distribution between Chloroplasts and Extrachloroplastic Space under Excess or Deficiency of Sulfate, Phosphate, or Magnesium. Plant Physiol. 1988 Aug;87(4):828–832. doi: 10.1104/pp.87.4.828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watad A. E., Pesci P. A., Reinhold L., Lerner H. R. Proton Fluxes as a Response to External Salinity in Wild Type and NaCl-Adapted Nicotiana Cell Lines. Plant Physiol. 1986 Jun;81(2):454–459. doi: 10.1104/pp.81.2.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watad A. E., Reinhold L., Lerner H. R. Comparison between a Stable NaCl-Selected Nicotiana Cell Line and the Wild Type : K, Na, and Proline Pools as a Function of Salinity. Plant Physiol. 1983 Nov;73(3):624–629. doi: 10.1104/pp.73.3.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyse R. E., Zamski E., Tomos A. D. Turgor regulation of sucrose transport in sugar beet taproot tissue. Plant Physiol. 1986 Jun;81(2):478–481. doi: 10.1104/pp.81.2.478. [DOI] [PMC free article] [PubMed] [Google Scholar]


