Abstract
Background:
The immune response to acute cerebral ischemia is a major factor in stroke pathobiology. Circadian biology modulates some aspects of immune response. The goal of this study is to compare key parameters of immune response during the active/awake phase versus inactive/sleep phase in a mouse model of transient focal cerebral ischemia.
Methods:
Mice were housed in normal or reversed light cycle rooms for 3 weeks, and then they were blindly subjected to transient focal cerebral ischemia. Flow cytometry was used to examine immune responses in blood, spleen and brain at 3 days after ischemic onset.
Results:
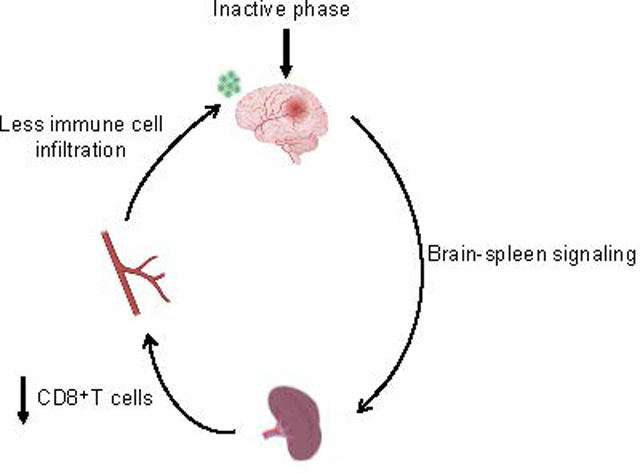
In blood, there were higher levels of circulating T cells in mice subjected to focal ischemia during zeitgeber time ZT1–3 (inactive or sleep phase) versus ZT13–15 mice (active or awake phase). In the spleen, organ weight and immune cell numbers were lower in ZT1–3 versus ZT13–15 mice. Consistent with these results, there was an increased infiltration of activated T cells into brain at ZT1–3 compared to ZT13–15.
Conclusions:
This proof-of-concept study indicates that there are significant diurnal effects on the immune response after focal cerebral ischemia in mice. Hence, therapeutic strategies focused on immune targets should be re-assessed to account for the effects of diurnal rhythms and circadian biology in nocturnal rodent models of stroke.
Keywords: circadian, diurnal, immune response, infiltrated cells, stroke
Graphical Abstract
INTRODUCTION
Circadian biology modulates all aspects of mammalian physiology and disease 1. Recently, it was hypothesized that these diurnal or daily rhythms may affect stroke mechanisms and neuroprotection in rodent models of cerebral ischemia 2. Because rodents are nocturnal, preclinical stroke studies that are performed in the daytime correspond to their inactive or sleep phase. In contrast, humans are diurnal and so the daytime corresponds to their active or awake phase 3. Hence, it is possible that there is a “circadian mismatch” between mechanisms and targets defined in preclinical rodent models versus the majority of active phase human strokes 4, 5.
Immune responses and inflammation play a major role in the pathophysiology of stroke 6. The immune system is modulated by diurnal and circadian rhythms 7. Diurnal and circadian effects on immunity have been shown to contribute to outcomes after cardiac ischemia 8. Therefore, it is likely that the immune reactions in stroke may also be influenced by the time-of-day of stroke onset. In this proof-of-concept study, we compare immune response in mice subjected to focal cerebral ischemia during the beginning of their inactive/sleep phase (zeitgeber ZT1–3) versus the beginning of their active/awake phase (ZT13–15).
METHODS
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.
Focal Cerebral Ischemia:
Male (C57BL/6J, 27–30g) (Jackson, Bar Harbor, ME) mice were housed in normal or reversed light cycle rooms for 3 weeks, and then all experiments were blindly performed during the daytime to correspond to either inactive (ZT1–3) or active (ZT13–15) phases 2. Experiments were in accordance with the National Institute of Health’s Guide for the Care and Use of Laboratory Animals, following Animals in Research: Reporting In vivo Experiments guidelines. Transient 60 min focal ischemia was induced introducing a 6–0 surgical monofilament nylon suture (Doccol). Infarction volumes based on 2,3,5-triphenyltetrazolium chloride (TTC) staining were blindly quantified using the “indirect” method with Image J software.
Flow Cytometry:
Single-cell suspensions prepared from blood, spleen or brain tissues were stained with fluorochrome conjugated antibodies. Antibodies were directly labeled with one of the following fluorescent tags: fluorescein isothiocyanate (FITC), phycoerythrin (PE), allophycocyanin (APC), PerCP-Cy5.5, PE-Cy7 or pacific blue. Cell phenotypes were performed on a FACS FORTESSA flow cytometer (BD Bioscience, Franklin lakes, NJ, USA). Data were blindly analyzed with Flow Jo software version 7.6.1 (Flow J, LLC, Ashland, OR, USA). Group differences were analyzed with 2-tailed unpaired t-test or 2-way ANOVA with P<0.05 as the threshold for significance. Please see Data Supplement for detailed procedures, analysis, and numerical values for individual cell types.
RESULTS
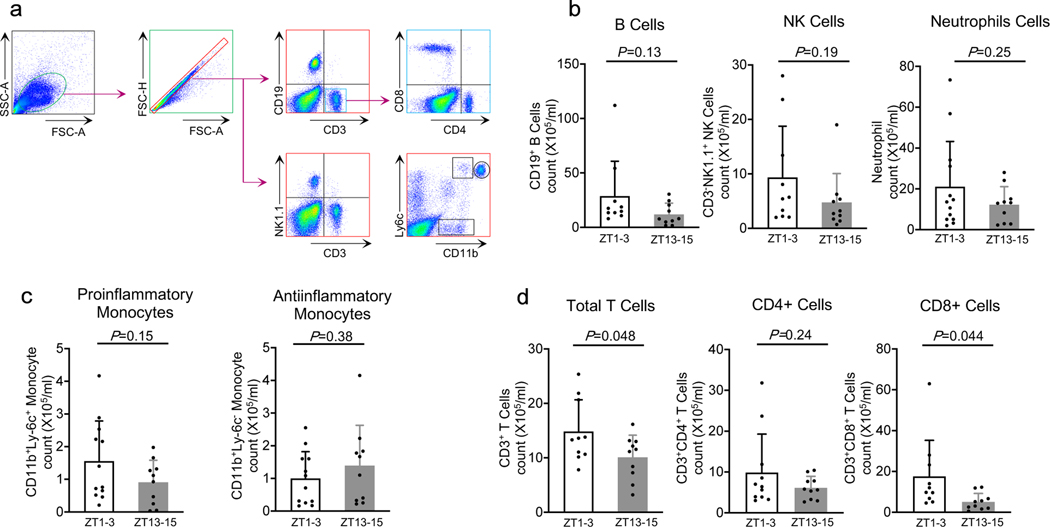
To assess immune response post-stroke, we collected circulating blood samples from all mice at 3 days post-ischemia. Flow cytometry analysis (Figure 1a) showed that there were relatively large variances in B cells, NK cells, neutrophils, and monocytes, and there were no statistically significant differences in response in ZT1–3 versus ZT13–15 mouse models (Figure 1b–c). However, circulating levels of T cells, especially activated CD8-positive T cells, were significantly higher in the inactive/sleep phase (ZT1–3) versus active/awake phase (ZT13–15) (Figure 1d) (n=10–12 per group).
Figure 1. Effects of diurnal rhythm in blood.
a. Gating strategy. b. Counts of CD19+ B cells (CD19+), NK cells (CD3-NK1.1+), neutrophils (CD11bhighLy-6c+). c. Ly6c+ monocytes (CD11b+Ly-6c+) or Ly6c- monocytes (CD11b+Ly-6c-). d. Numbers of total T cells (CD3+), CD4+ T cells (CD3+CD4+) and CD8+ T cells (CD3+CD8+). (n=10–13). All values are mean±SD; comparisons via 2-tailed t-test.
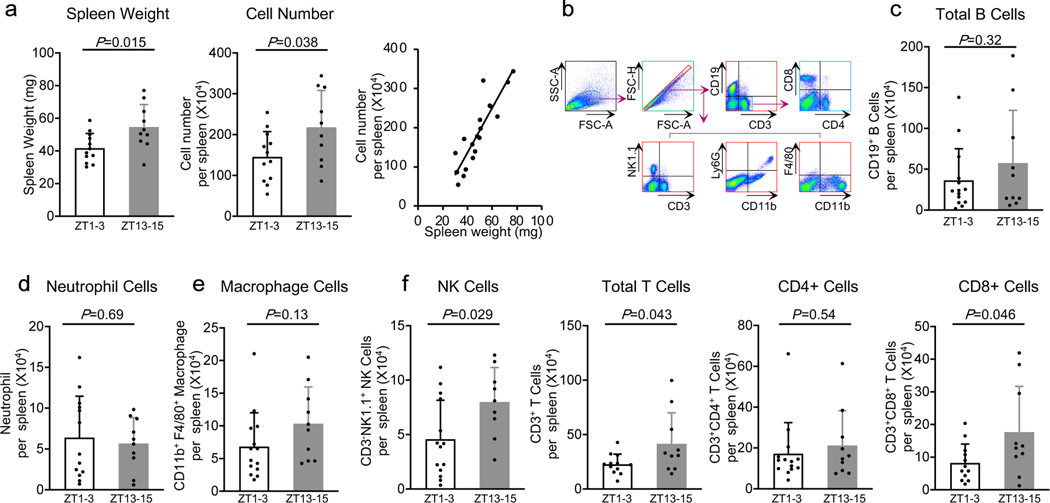
Reductions in spleen size are known to occur after stroke. At 3 days after focal cerebral ischemia, spleen weight and total cell numbers were smaller in mouse models performed during ZT1–3 compared to ZT13–15 (Figure 2a) (n=10–12 per group). By flow cytometry (Figure 2b), there were no detectable differences in spleen populations of B cells, neutrophils and macrophages (Figure 2c–e). However, the numbers of NK cells and total T cells were lower in spleens obtained from ZT1–3 versus ZT13–15 mouse models. The differences in T cells appeared to be mostly driven by differences in activated CD8-positive T cells (Figure 2f) (n=9–14 per group).
Figure 2. Effects of diurnal rhythm in spleen.
a. Spleen weight, total cell numbers and their correlation (n=10–12). b. Gating strategy. c-e. Counts of B cells (CD19+), neutrophils (CD11b+Ly-6G+) and macrophages (CD11b+ F4/80+). f. Numbers of total T cells (CD3+), CD4+ T cells (CD3+CD4+), CD8+ T cells (CD3+CD8+) and NK cells (CD3-NK1.1+). (n=9–14). All values are mean±SD; comparisons via 2-tailed t-test.
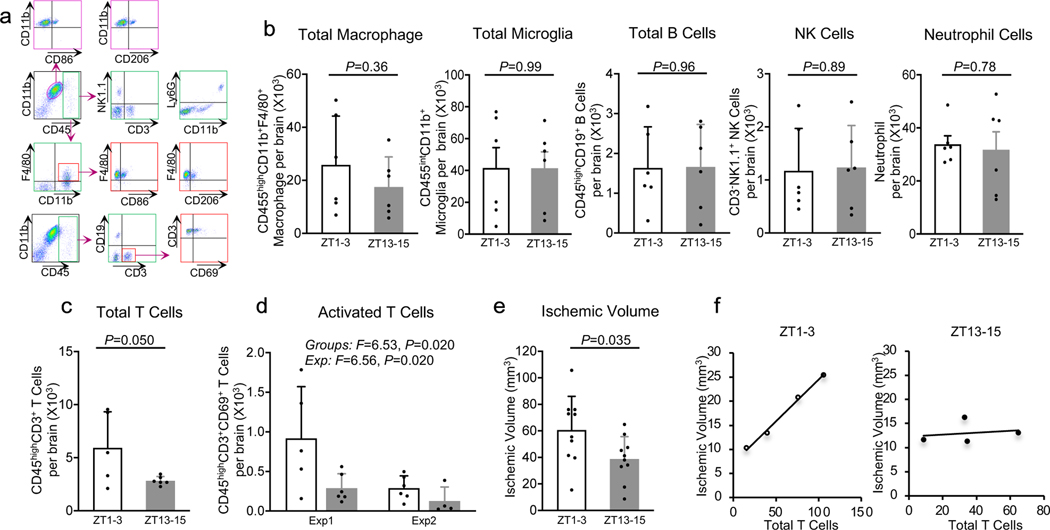
Next, we asked whether these systemic differences in immune response might also be accompanied by differences in brain infiltration of immune cells after cerebral ischemia. Flow cytometry of brain tissue at 3 days after focal ischemia (Figure 3a) showed that the total numbers of macrophages and microglia were comparable in ZT1–3 and ZT13–15 models. There were no differences in B cells, NK cells and neutrophils (Figure 3b, Supplementary Data, n=5–6 per group). However, the number of infiltrating T cells were higher in mice subjected to ischemia during ZT1–3 compared with mice subjected to ischemia during ZT13–15 (Figure 3c). To further assess these findings, we performed a replication experiment (n=4–6 per group). Although there were overall differences in the magnitude of ischemic immune response between these two independent experiments performed almost one year apart, 2-way ANOVA analysis confirmed that activated T cells in brain were indeed higher in mouse models of focal cerebral ischemia during the inactive/sleep phase (ZT1–3) compared with the active/awake phase (ZT13–15) (Figure 3d).
Figure 3. Effects of diurnal rhythm in brain.
a. Gating strategy. b. Numbers of macrophage (CD45highCD11b+F4/80+), microglia (CD45intCD11b+), B cells (CD45highCD19+), NK cells (CD3-NK1.1+) and neutrophils (CD11b+CD45highLy-6G) (n=5–6). c. Counts of T cells (CD45highCD3+) (n=5–6). d. Counts of CD69 expressing T cells (CD45highCD3+ CD69) from exp1 and exp2 (n=5–6 for each exp) e. Ischemic volume (n=10). f. Correlation between ischemic volume and T cells (n=4). All values are mean±SD; comparisons via 2-tailed t-test or 2-way Anova.
Finally, we asked whether these differences in immune response were accompanied by differences in brain infarction. Tetrazolium staining showed that 3-day infarct volumes were larger when focal cerebral ischemia was induced in mice during the inactive/sleep phase (ZT1–3) compared to the active/awake phase (ZT13–15) (Figure 3e) (n=10 per group). There was a correlation between infarct volume and blood levels of T cells in mice subjected to focal cerebral ischemia during their inactive/sleep phase ZT1–3 (R2=0.99, p=0.003) but not their active/awake phase ZT13–15 (R2=0.04, p=0.798) (Figure 3f) (n=4).
DISCUSSION
At 3 days after focal cerebral ischemia in mice, T cell levels in blood and recruitment into brain are higher for inactive phase ZT1–3 models compared with active phase ZT13–15 models. In the spleen, an opposite pattern was observed, with a lower tissue mass and reduced number of resident T cells in inactive phase ZT1–3 models compared with active phase ZT13–15 models. Rodent spleens are known to decrease in size after focal cerebral ischemia, and there is a negative correlation between spleen size and infarct volume 9. Hence, these data altogether suggest that during inactive phase strokes, there may be greater recruitment of immune cells to move out from the spleen into the circulation and home towards ischemic brain at the time studied. There was a correlation between blood levels of T cells and infarct volumes for mice subjected to ischemia during their inactive/sleep phase, consistent with the hypothesis of immune cells participating in secondary brain injury after stroke. However, there was no correlation during their active/awake phase. Therefore, the differences in immune response observed here may not just be indirectly due to larger or smaller infarcts in ZT1–3 versus ZT13–15 mouse models. Furthermore, the diurnal differences in immune response at 72 h did not extend across all cell types, and no significant differences were noted in macrophages, neutrophils and B cells. Within the limits of our relatively small group numbers and time windows, we did not detect statistically significant differences in non-ischemic immune baselines between ZT1–3 versus ZT13–15 blood and spleen samples (Supplementary Data; n=6 per group), suggesting that the differences observed after our stroke model might be due to a response to ischemia. Nevertheless, it is known that some subsets of immune cells show a diurnal cycle 7. Therefore, the question of causal mechanisms will be especially important, i.e. whether diurnal differences in systemic immune cells leads to differences in brain injury, or whether diurnal differences in other mechanisms underlying ischemic brain injury induces subsequent differences in immune response, or both. Future studies are warranted to dissect the mechanisms underlying the interaction between diurnal rhythms and immune response after stroke.
Taken together, our results suggest that immune responses in mouse models of focal cerebral ischemia are indeed different depending on the time-of-day of stroke onset. However, several caveats must be kept in mind. First, although our flow cytometry analyses covered major immune cell categories, detailed responses and potential involvement of smaller subsets remain to be mapped. It may be useful to use high resolution CYTOF-based techniques 10 to fully characterize the diurnal modulation of stroke-induced immune response. Second, we only measured immune cells at 3 days, therefore we may have missed differences at other timepoints. For example, neutrophil infiltration can occur by 24 hrs and T-regs can continue to accumulate up to a week. Future studies should analyze daily rhythms across the full time-course of immune reactions post-stroke 6. Third, diurnal and circadian rhythms are affected by stroke itself 11. The regulation of feed-forward and feed-back signals between stroke and daily rhythms in various aspects of physiology should be examined. Finally, we only tested young male mice. Both diurnal rhythms and immune biology are affected by age and sex 12. How these pathways may differ in older or female mouse models remain to be determined.
In conclusion, this proof-of-concept study suggests that there are differences in immune response in strokes that occur during day versus night. From a translational perspective, further studies are warranted to examine how immune mechanisms and targets currently defined for inactive/sleep phase models in nocturnal rodents are modified when re-examined in active/awake phase models that should better match the majority of human daytime strokes.
Supplementary Material
Acknowledgments:
Supported in part by the Rappaport Foundation and Leducq Foundation. The authors thank all team members of the MGH animal facility for help with light schedule switching.
ABBREVIATIONS:
- CYTOF
cytometry by time-of-flight
- TTC
2,3,5-triphenyltetrazolium chloride
- ZT
zeitgeber time
Footnotes
Disclosures: None.
REFERENCES
- 1.Adrover JM, Del Fresno C, Crainiciuc G, Cuartero MI, Casanova-Acebes M, Weiss LA, Huerga-Encabo H, Silvestre-Roig C, Rossaint J, Cossío I, et al. A neutrophil timer coordinates immune defense and vascular protection. Immunity. 2019;51:966–967 [DOI] [PubMed] [Google Scholar]
- 2.Esposito E, Li W, Mandeville TE, Park JH, Sencan I, Guo S, Shi J, Lan J, Lee J, Hayakawa K et al. Potential circadian effects on translational failure for neuroprotection. Nature. 2020;582:395–398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lo EH, Albers GW, Dichgans M, Donnan G, Esposito E, Foster R, Howells DW, Huang YG, Ji X, Klerman EB, et al. Circadian biology and stroke. Stroke. 2021;52:2180–2190 [DOI] [PubMed] [Google Scholar]
- 4.Boltze J, Didwischus N, Merrow M, Dallmann R, Plesnila N. Circadian effects on stroke outcome - did we not wake up in time for neuroprotection? J Cereb Blood Flow Metab. 2021;41:684–686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zaidat OO, Changal KH, Sultan-Qurraie A, de Havenon A, Calderon VJ, Goins-Whitmore J,. Patterson SM, Lin E. Diurnal variations in the first 24/7 mobile stroke unit. Stroke. 2019;50:1911–1914 [DOI] [PubMed] [Google Scholar]
- 6.Garcia-Culebras A, Duran-Laforet V, Pena-Martinez C, Ballesteros I, Pradillo JM, Diaz-Guzman J, Lizasoain I, Moro MA. Myeloid cells as therapeutic targets in neuroinflammation after stroke: Specific roles of neutrophils and neutrophil-platelet interactions. J Cereb Blood Flow Metab. 2018;38:2150–2164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scheiermann C, Gibbs J, Ince L, Loudon A. Clocking in to immunity. Nat Rev Immunol. 2018;18:423–437 [DOI] [PubMed] [Google Scholar]
- 8.Reitz CJ, Alibhai FJ, Khatua TN, Rasouli M, Bridle BW, Burris TP, Martino TA. Sr9009 administered for one day after myocardial ischemia-reperfusion prevents heart failure in mice by targeting the cardiac inflammasome. Commun Biol. 2019;2:353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seifert HA, Offner H. The splenic response to stroke: From rodents to stroke subjects. J Neuroinflammation. 2018;15:195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kott KA, Vernon ST, Hansen T, de Dreu M, Das SK, Powell J, Fazekas de St Groth B, Di Bartolo BA, McGuire HM, Figtree GA. Single-cell immune profiling in coronary artery disease: The role of state-of-the-art immunophenotyping with mass cytometry in the diagnosis of atherosclerosis. J Am Heart Assoc. 2020;9:e017759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hasan F, Gordon C, Wu D, Huang HC, Yuliana LT, Susatia B, Marta OFD, Chiu HY. Dynamic prevalence of sleep disorders following stroke or transient ischemic attack: Systematic review and meta-analysis. Stroke. 2021;52:655–663 [DOI] [PubMed] [Google Scholar]
- 12.Fischer D, Lombardi DA, Marucci-Wellman H, Roenneberg T. Chronotypes in the us - influence of age and sex. PLoS One. 2017;12:e0178782 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.