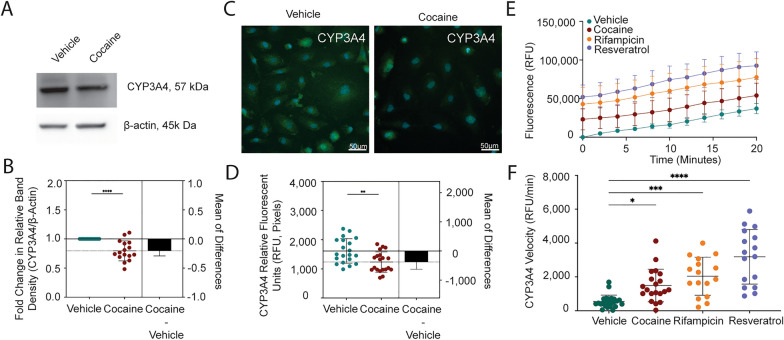
Fig. 9.
Cocaine Decreases CYP3A4 to Compensate for Increased Enzymatic Activity. A Western blot was performed to evaluate CYP3A4 following 24-h treatment with cocaine (10 μM) or vehicle. β-actin was used for protein normalization. One western blot, representative of 16 independent experiments, is shown. B The fold change in relative band intensity for CYP3A4/β-actin was determined by densitometry where vehicle treatment was set to 1. Sixteen independent experiments (represented by individual dots) were performed. The fold change in relative band intensity for CYP3A4 relative to β-actin is depicted (bottom). Data are represented as mean ± standard deviation. *p < 0.05. One-way ANOVA was performed. C Immunofluorescent microscopy was performed to evaluate CYP3A4 (green) following treatment with cocaine (10 μM, right) or vehicle (left) for 24 h. DAPI was used to visualize the nucleus (blue). One paired representative image, out of 20 individual images, is shown. All scale bars = 50 μm. D Quantification of the fluorescent signal from CYP3A4 immunofluorescent microscopy was performed for endothelial cells treated with cocaine (10 μM, burgundy) or vehicle (teal) for 24 h. Twenty independent experiments (represented by individual dots) were performed. Estimation plots are shown where the left y-axis denotes relative fluorescent intensity (RFU, pixels) and the right y-axis reflects the effect size (black bar), which is the difference between means of each condition. Data are represented as mean ± standard deviation. **p < 0.01. Unpaired T-test was performed. E Endothelial cells were pre-treated with cocaine (10 μM, burgundy), rifampicin (1 μM, yellow), resveratrol (10 μM, lavender), or vehicle (teal) for 24 h, after which time the cells were loaded with BFC (2 μM). The enzymatic capacity of CYP3A4 to convert BFC to HFC was determined for the first 20 min as determined by fluorometric quantitation at excitation and emission wavelengths of 405/535 nm. Twelve independent experiments that contained eight technical replicates per condition were performed. Data are represented as mean ± standard deviation. **p < 0.01. ****p < 0.0001. Unpaired T-test was performed. F The rate at which BFC was converted to HFC is depicted as CYP3A4 velocity (RFU/min) for the earliest time points (2 and 4 min) to evaluate maximal enzymatic activity. The CYP3A4 velocity for each time point was pooled for both time points. Twelve independent experiments for each time point (represented by combined 24 individual dots) were performed. Data are represented as mean ± standard deviation. *p < 0.05. ***p < 0.001. ****p < 0.0001. One-way ANOVA was performed