Abstract
Simple Summary
Forty percent of patients with diffuse large B-cell lymphoma (DLBCL) have a refractory or relapsed (R/R) disease. In this setting, prognosis is poor, particularly for patients not eligible for autologous stem-cell transplantation or CAR-T-cell therapy, thus representing an unmet need in the field of hematological malignancies. Currently, the optimal treatment approach for these patients remains controversial. Over the last few decades, monoclonal antibodies (mAbs) have dramatically changed the therapeutic landscape for cancer patients. The aim of our review is focused on novel and emerging therapeutic strategies based on different types of mAbs, including monospecific and bispecific mAbs as well as antibody–drug conjugates and immune checkpoint inhibitors, in the challenging setting of R/R DLBCL.
Abstract
Diffuse large B-cell lymphoma (DLBCL) is the most common aggressive lymphoma. Approximately 60% of patients are cured with R-CHOP as a frontline treatment, while the remaining patients experience primary refractory or relapsed disease (R/R). The prognosis for R/R DLBCL patients who are neither eligible for autologous stem-cell transplantations nor CAR-T-cell treatment is poor, representing an important unmet need. Monoclonal antibodies (mAbs) have dramatically improved therapeutic options in anti-cancer strategies, offering new opportunities to overcome chemo-refractoriness in this challenging disease, even in cases of primary non-responder DLBCL. Several novel mAbs, characterized by different mechanisms of action and targets, are now available for R/R DLBCL. Unbound mAbs induce an immune response against cancer cells, triggering different mechanisms, including antibody-dependent cellular cytotoxicity (ADCC), activation of antibody-dependent cell-mediated phagocytosis (ADCP) and complement-dependent cytotoxicity (CDC). Antibody–drug conjugates (ADCs) and radioimmunotherapy (RIT), respectively, deliver a cytotoxic payload or a beta-emitter radionuclide to the targeted cells and nearby bystanders. Bispecific T-cell engagers (BiTes) and immune checkpoint inhibitors (ICIs) redirect and enhance the immune response against tumor cells. Here, we review therapeutic strategies based on monoclonal antibodies for R/R DLBCL.
Keywords: diffuse large B-cell lymphoma, monoclonal antibodies, target therapy, bispecific antibodies, antibody-dependent cellular cytotoxicity, immune checkpoint inhibitors
1. Introduction
Diffuse large B-cell lymphoma (DLBCL) is an aggressive lymphoma and the most common subtype of non-Hodgkin’s lymphoma (NHL), accounting for approximately 30–40% of all diagnoses of NHL. The prevalence is higher in low/middle-income countries (~42.5%) than in high-income countries (~28.9%), with a median age at diagnosis of 53 and 65 years, respectively [1,2,3]. Most diagnoses are found in previously healthy patients, without any history of hematological malignancies, though a fraction of cases may arise from the transformation of a previous low-grade B-cell lymphoma.
The introduction of the anti-CD20 monoclonal antibody (mAb) rituximab in the CHOP regimen (R-CHOP) significantly improved the cure rate to 60% [4,5]. No other regimen, including intensified chemoimmunotherapy [6,7,8], use of second-generation anti-CD20 mAb [9], maintenance therapy [10,11,12] or targeted drugs [13,14,15,16,17], proved to be superior to R-CHOP in terms of overall survival (OS) and progression-free survival (PFS). Recently, pola-R-CHP, in which vincristine was replaced with the anti-CD79b mAb polatuzumab vedotin, has been proven to lower the risk of disease progression in previously untreated DLBCL [18].
Forty percent of patients have a refractory or relapsed disease (R/R DLBCL). Primary refractory disease (10–15% of cases) is defined as a lack of response to treatment, whereas relapsed disease (20–30% of cases), which usually occurs within the first 2 years from the end of treatment, is defined by the appearance of new lesions after achieving a complete response [19,20]. The prognosis for R/R DLBCL is very poor, particularly for primary refractory disease or early relapse (relapse within 3 to 6 months), in which the median OS is approximately 6 months [21]. The salvage regimen in transplantable-eligible patients relies on a rituximab-based chemoimmunotherapy followed by autologous stem-cell transplantation (ASCT). However, only 60% of these patients obtain a sustained remission with a 4-year progression-free survival (PFS) and OS, respectively, of 56% and 65% and an event-free survival (EFS) of 30% [22]. Most R/R DLBCL patients, however, are not ASCT eligible due to age, comorbidities or an inadequate response to salvage chemoimmunotherapy.
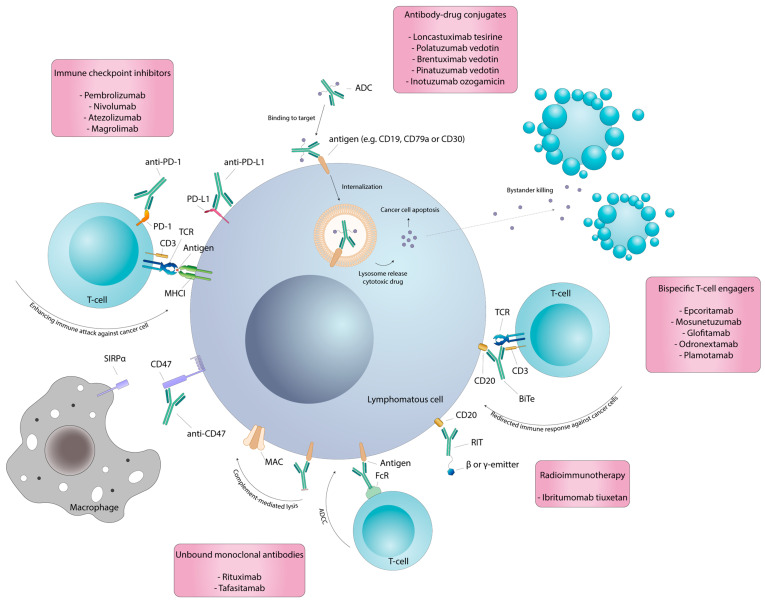
During the last few years, the advent of new treatments has provided alternatives to conventional therapies, in many cases obtaining sustained remission and survival improvements. The aim of this review is to provide a focus on novel mAbs in the setting of R/R DLBCL (Figure 1).
Figure 1.
This figure illustrates the mechanisms of action of therapeutic monoclonal antibodies. The binding of an unbound monoclonal antibody to its antigen induces an immune response against targeted cancer cells through antibody-dependent cellular cytotoxicity, antibody-dependent cell-mediated phagocytosis and complement-dependent cytotoxicity. Internalization of antibody–drug conjugates into cancer cells leads to tumor cell death due to the release of cytotoxins. Following apoptosis of the targeted cancer cells and diffusion in the extracellular space, these cytotoxins can promote bystander killing. Similarly, a monoclonal antibody conjugated to a radionuclide delivers radioactive particles to targeted tumor cells as well as nearby tumor cells, resulting in their death. Blocking immune escape mechanisms, such as PD-1/PD-L1 and CD47/SIRPα signaling, restores an appropriate immune response against tumor cells. Bispecific T-cell engagers bind both tumor cells and T cells, redirecting a selective cytotoxic response against cancer cells. ADC: antibody–drug conjugates; TCR: T-cell receptor; MHCI: major histocompatibility complex class I; BiTe: bispecific T-cell engager; RIT: radioimmunotherapy; MAC: membrane attack complex; FcR: Fc receptor.
2. Monoclonal Antibodies
During the last few decades, numerous advances in molecular biology and in biotechnologies have significantly improved both the available number of anti-cancer drugs and therapeutic strategies, according to Paul Ehlrich’s forward-looking concept of “magic bullet” formulated more than one hundred years ago. Such a concept became available particularly thanks to the introduction of hybridoma technology [23], which provided the knowledge needed to develop mAbs capable of high selectivity towards their antigens.
Therapeutic mAbs, either chimeric or humanized, generally belong to the γ-immunoglobulin (IgG) isotype. The binding of the unbound antibody to its antigen induces an immune response against the targeted cell through antibody-dependent cellular cytotoxicity (ADCC), the activation of antibody-dependent cell-mediated phagocytosis (ADCP) and complement-dependent cytotoxicity (CDC). Another strategy relies on mAbs linked to cytotoxic drugs (antibody–drug conjugates, ADCs) or radionuclides (radioimmunotherapy, RIT) that, once bound to their target, selectively deliver their payload to cancer cells [24]. Bispecific T-cell engagers (BiTes) are mAbs capable of recognizing a tumor-associated antigen and the invariant component of the T-cell receptor (TCR) complex, CD3, redirecting a selective T-cell response against tumor cells [25]. Cancer cells can hijack immunological surveillance by overexpressing immunoinhibitory molecules (e.g., PD-L1). The blockade of these molecules or their receptors on cytotoxic lymphocytes reactivates T cells against cancer cells. This is the mechanism of action of mAbs known as immune checkpoint inhibitors [26]. Thus, both BiTes and immune checkpoint inhibitors enhance and redirect the immune response against cancer cells. Many of these mAbs have provided proof of efficacy in R/R DLBCL and have received FDA and/or EMA approval, and several others are currently under investigation or development.
3. Unbound Monoclonal Antibodies
Tafasitamab is an Fc-enhanced, humanized mAb that targets CD19, an antigen broadly and homogenously expressed by B-cell malignancies, including DLBCL. The Fc-enhancement consists of the introduction of two amino acid substitutions (S239D and I332E) within the Fc region that increase the affinity for Fcγ receptors on immune effector cells. The binding of tafasitamab to the CD19 antigen activates ADCC and ADCP and leads to tumor cell killing [27,28].
One of the first studies to evaluate the tolerability and efficacy of tafasitamab enrolled patients with R/R chronic lymphocytic leukemia (CLL) and demonstrated a safe profile and initial proof of clinical activity [29]. Afterwards, Jurckzac et al. evaluated tafasitamab as monotherapy in B-NHLs, highlighting the objective responses in R/R DLBCL and indolent lymphomas (26% and 29%, respectively), including rituximab-refractory diseases. Tafasitamab was well tolerated, with neutropenia as the most common adverse event (AE), especially in the heavily pretreated subgroup of DLBCL, and few grade 3 or 4 non-hematological AEs (mainly dyspnea and pneumonia) [27]. Preclinical data have shown a potential synergy of tafasitamab and lenalidomide [27], an immunomodulatory, antineoplastic and antiangiogenic agent that stimulates the proliferation and activation of NK cells, thus enhancing ADCC [30]. Lenalidomide, though, has limited clinical activity in R/R NHLs as a single agent [31,32,33,34], with a range of objective responses from 28% to 35% [28]. L-MIND, a multicenter, open-label, single-arm, phase 2 study, investigated the activity and safety of the lenalidomide and tafasitamab combination in R/R DLBCL ineligible for ASCT. The ORR was 60% (CR 43%), with a median duration of response (DOR) of 21.7 months and an 18-month PFS of 46%. The most common grade ≥ 3 AEs were thrombocytopenia, febrile neutropenia, leukopenia, anemia and pneumonia, while non-hematological AEs were of low grades (diarrhea and rash). Importantly, the incidence and severity of treatment-emergent AEs decreased after the discontinuation of lenalidomide, suggesting a main role in toxicity [28]. The long-term outcomes of the updated analysis with more than 35 months of follow-up were consistent in terms of tolerability and efficacy, with an ORR of 57.5% (CR 40%, PR 17.5%), an mDOR of 43.9 months, an mOS of 33.5 months and an mPFS of 11.6 months. The mPFS and mOS were shorter in primary refractory patients and in patients with an IPI score ≥ 3 (intermediate–high or high risk) [35]. Based on these data, tafasitamab in combination with lenalidomide was granted accelerated approval by the FDA in 2020 and conditional/accelerated approval by the EMA in 2021. Further data on patients who received treatment for ≥2 years and those in follow-up for ≥5 years confirmed that this combination provides durable responses with a decreased incidence of AEs during tafasitamab monotherapy [36]. The firmMIND trial is an ongoing, phase 3, post-authorization commitment study with the aim to confirm the efficacy, safety and overall benefit/risk of the combination in R/R DLBCL [37].
Following the approval by the FDA and EMA, many combination and comparison trials on tafasitamab in DLBCL have been performed. RE-MIND2 is a real-world study comparing the efficacy of tafasitamab plus lenalidomide (from L-MIND trial) versus other systemic anti-DLBCL regimens, including polatuzumab vedotin plus bendamustine-rituximab (pola-BR), rituximab-lenalidomide (R2) and CAR-T therapies, using a retrospective, observational, matched-cohort approach. This analysis was based on propensity score matching, which extrapolated and compared comparable populations from two different trials. Considering the limitations of this statistical approach, a significant difference in OS, favoring tafasitamab plus lenalidomide over the pola-BR and R2 cohorts, was observed, while OS appeared comparable to that of the CAR-T cohort [38]. Via matching-adjusted indirect comparisons (MAICs) of tafasitamab + lenalidomide using data from L-MIND, tafasitamab + lenalidomide was associated with a significantly improved OS, PFS, DOR and CR compared to bendamustine-rituximab (BR) [39]. B-MIND is a phase 2/3, randomized, multicenter study focused on the administration of tafasitamab with bendamustine versus BR in R/R DLBCL not eligible for ASCT (NCT02763319), whose results are ongoing. The combination of tafasitamab with different novel agents, such as plamotamab (NCT05328102) or selinexor (NCT04607772), is currently under investigation. Finally, tafasitamab plus lenalidomide is being tested as a frontline treatment in newly diagnosed DLBCL. The firstMIND and frontMIND trials explore the comparison and association of tafasitamab plus lenalidomide and R-CHOP as the first line in DLBCL, based on the treatment strategy concept that targeting both CD19 and CD20 on B tumor cells may limit target evasion in patients with low CD20 expression (Table 1) [40,41]. In conclusion, the combination of tafasitamab and lenalidomide appears to be a successful immunotherapy option in a population of heavily pretreated patients that, until recently, lacked a valid alternative to palliative treatment. A head-to-head comparison with the other novel therapies (such as CAR-T and other types of mAbs) is needed to understand the differences in terms of survival and toxicity.
Table 1.
Unbound monoclonal antibodies clinical trials.
| NCT | Phase | Targets | Drugs | Results |
|---|---|---|---|---|
| NCT05626322 | phase II | CD47, CD19 | maplirpacept-tafasitamab-lenalidomide | NA |
| NCT01685008 | phase IIa | CD19 | tafasitamab | ORR 26%, CR 6%; mPFS 2.7 months, mDOR 20.1 months |
| NCT02399085 | phase II | CD19 | tafasitamab-lenalidomide | ORR 60%, CR 40%; mPFS 18 months in 46%, mDOR 21.7 months |
| NCT05429268 | phase III | CD19 | tafasitamab-lenalidomide | NA |
| NCT04697160 | real world | CD19 | tafasitamab-lenalidomide vs. other therapies (R2, pola-BR, CAR-T) |
ORR 62.5% vs. 58.3% (pola-BR); 63.6% vs. 30.3% (R2); 59.5% vs. 75.7% (CAR-T) |
| NCT02763319 | phase II/III | CD19 | tafasitamab-bendamustine vs. rituximab-bendamustine |
NA |
| NCT05328102 | phase II | CD19, CD20/CD3 | tafasitamab-lenalidomide-plamotamab vs. tafasitamab-lenalidomide |
NA |
| NCT04607772 | phase I/II | CD19, XPO1 | selinexor-tafasitamab-lenalidomide vs. selinexor-other therapies |
NA |
R2: lenalidomide-rituximab; pola-BR: polatuzumab-bendamustine-rituximab; CAR-T: chimeric antigen receptor-T cells; ORR: overall response rate; CR: complete response; mPFS: median progression-free survival; mDOR: median duration of response; NA: not available.
4. Bispecific Antibodies
Bispecific antibodies are emerging as a promising off-the-shelf option for R/R DLBCL. These mAbs bind both to tumor cells and to T cells, redirecting a selective cytotoxic response against tumor cells [25].
Epcoritamab (GEN3013) is a bispecific IgG1 antibody that targets CD3 and CD20, respectively, on T and B cells, therefore activating and redirecting T cells towards B cells and causing their cytotoxicity-mediated death [42]. Epcoritamab induced effective and comparable levels of cytotoxicity in DLBCL, follicular lymphoma (FL) and mantle cell lymphoma (MCL) and it was effective in samples from newly diagnosed and R/R patients. Importantly, this evidence included patients previously exposed to other anti-CD20 mAbs, indicating that epcoritamab can be administered shortly after anti-CD20 exposure [43]. EPCORE NHL-1 (NCT03625037) was the first-in-human phase I/II trial exploring the dose escalation and dose expansion of subcutaneous epcoritamab in R/R CD20-positive NHL previously treated with an anti-CD20 mAb. In R/R DLBCL, the ORR was 88% (CR of 38%) with objective responses also achieved after CAR-T therapy [44]. No treatment-related AEs led to discontinuation or death, and all cytokine release syndrome (CRS) events were lower than grade 3 and managed with standards of care. CRSs were 49.7% (grade 1 or 2: 47.1%; grade 3: 2.5%), pyrexia 23.6%, fatigue 22.9% and ICANS 6.4% [45]. The favorable activity of a single-agent epcoritamab in such a difficult-to-treat population led to the development of further clinical trials, in monotherapy or in combinations with standard therapies, most of which are still ongoing (NCT04663347, NCT05852717, NCT04628494, NCT05578976) [46].
Mosunetuzumab is a full-length, humanized, IgG1 bispecific antibody targeting CD3 and CD20. The first-in-human dose-escalation study of a single-agent mosunetuzumab showed an ORR of 34.9% (CR 19.4%) in B-NHL. CRS was the most common toxicity in 27.4% of patients, most of which were grade 1 and 2. Other toxicities included neutropenia, hypophosphatemia, anemia and low-grade neurologic events [47]. Subsequent phase I/Ib and phase II trials documented a similar benefit–risk profile, highlighting the better rates of responses in indolent versus aggressive NHL (ORR and CR, respectively, 64.1% vs. 34.7% and 42.2% vs 18.6%), also achieving objective responses in patients previously exposed to CAR-T [48,49]. The fixed-duration regimen of mosunetuzumab could potentially reduce the cumulative safety risk and appeared to be suitable for unfit, elderly or ASCT/CAR-T-ineligible patients. Mosunetuzumab is also available in subcutaneous formulation [49,50,51], which demonstrated a clinical activity comparable to the intravenous formulation (ORR 82% and CR 64% in indolent NHL; ORR 36% and CR 20% in aggressive NHL) [51]. The combination of mosunetuzumab with polatuzumab vedotin is currently under investigation (NCT03671018) [52]. More association trials are underway, including combination with other BiTes, as glofitamab in patients who previously received CAR-T (NCT04889716); with novel ADCs, such as loncastuximab tesirine (NCT05672251); and with historical salvage platinum-based immunochemotherapy in patients who are candidates for ASCT (NCT05464329), outlining a vast scenario of possible consecutive or coexisting treatments in continuous development.
At variance from other BiTes, glofitamab is a full-length bispecific antibody with a special 2:1 configuration allowing a bivalent binding to CD20 on B cells and a monovalent binding to CD3 on T cells and potentially leading to a superior potency compared to 1:1 bispecific antibodies. Administration of the anti-CD20 obinutuzumab before glofitamab successfully reduces the risk of CRS, acting on B-cell depletion and reducing T-cell activation, with a comparable antitumor activity compared to glofitamab alone [53]. NP30179 was the first-in-human phase I trial investigating an intravenous single-agent glofitamab after single-dose obinutuzumab (7 days before) and with ongoing co-administered obinutuzumab in B-NHLs. The combination led to an ORR of 71.4% (CR 64.3%) in aggressive NHL. The most common AEs included low-grade CRS, rare self-limiting ICANS-like events and cytopenia. Importantly, the preservation of glofitamab activity observed despite the presence of a CD20 receptor competitor, such as obinutuzumab, represents a unique advantage of this antibody [54]. A phase II trial with a fixed-duration glofitamab monotherapy (12 cycles in total) showed durable CRs in R/R DLBCL, also in patients previously exposed to CAR-T cells, with 78% of complete responses at 12 months [55]. This evidence opens the question whether bispecific antibodies need to be administered until progression or for a fixed course. The numerous subsequent investigations on real-world data [56,57] and on combinations with other novel mAbs [58] will confirm the durability of the response after glofitamab and clarify its role on heavily pretreated patients, especially in patients who received prior CAR-T. Furthermore, of special interest are the ongoing trials that explore the association or the comparison of glofitamab with standards of care (NCT04408638, NCT05364424, NCT05335018) and its single-agent use in untreated DLBCL [59].
Odronextamab (REGN1979) differs from other CD3xCD20 bispecific T-cell engagers because of the minimal engineering and native antibody structure, as it is fully human, IgG4-based and hinge-stabilized [60]. The pivotal phase I first-in-human trial ELM-1 investigated the dose escalation and dose expansion of intravenous odronextamab in indolent and aggressive B-NHLs, including those with prior CAR-T therapy. Encouraging antitumor activity was observed both in FL and R/R DLBCL. The safety profile was similar to that of other BiTes, with a low rate of severe (≥grade 3) CRS and ICANS-like events. In R/R DLBCL not previously exposed to CAR-T, the ORR was 53% with all complete responses, whereas in the CAR-T-exposed population, the ORR and CR were, respectively, 33% and 27%. Interestingly, the estimated probability of maintaining a CR at 12 months was 88% in CAR-T-naïve and 100% in CAR-T-exposed patients, highlighting the potential benefit in heavily pretreated patients [61]. The subsequent phase II study ELM-2 confirmed the ELM-1 results in both CAR-T-naïve and CAR-T-exposed R/R DLBCL, with an ORR of 53% and a CR of 37%; the responses appeared to be durable with a probability of ongoing CR at 9 months of 73% [62]. Odronextamab is also under investigation in a subcutaneous formulation (NCT02290951). Furthermore, in preclinical NHL models, odronextamab has been evaluated in association with another novel antibody bispecific for CD28xCD22, REGN5837, which provides a costimulatory signal for T cells, enhancing their activation and thus promoting odronextamab activity, opening the way for a possible combination use [63].
Plamotamab is the most recent CD3xCD20 BiTe and still under investigation in a phase I dose-escalation study (NCT02924402) [64,65]. B-NHL patients, including prior CAR-T-exposed patients (50%), received intravenous single-agent plamotamab in a dose-escalation study. The preliminary results showed a manageable toxicity profile, with CRS as the most common adverse events (all of grade 1–2) and severe AEs (grade 3 or 4) consisting in anemia (19.4%), neutropenia (16.7%) and thrombocytopenia (11.1%). In DLBCL, the ORR was 47.4% (CR 26.3%) and in those prior exposed to CAR-T, the ORR was 46.2% (CR 30.8%). Based on these findings, a combination study involving tafasitamab and lenalidomide plus plamotamab versus the approved tafasitamab and lenalidomide in R/R DLBCL has just completed enrollment. The addition of plamotamab might offer a potential improved efficacy through the recruitment of distinct T-cell and Fc-mediated cytotoxic mechanisms, while also overcoming tumor resistance that might otherwise arise from the single antigenic loss of either CD19 or CD20. The results are underway (NCT05328102) (Table 2).
Table 2.
Bispecific antibodies clinical trials.
| NCT | Phase | Target | Drug | Results |
|---|---|---|---|---|
| NCT03625037 | phase I/II | CD20/CD3 | epcoritamab | ORR 88%, CR 38% Expansion cohort: ORR 63.1%, CR 38.9% |
| NCT04663347 | phase Ib/2 | CD20/CD3 | epcoritamab + other therapies | NA |
| NCT05852717 | phase II | CD20/CD3 | epcoritamab + GDP | NA |
| NCT04628494 | phase III | CD20/CD3 | epcoritamab vs. investigator choice therapy | NA |
| NCT02500407 | phase I/Ib | CD20/CD3 | mosunetuzumab | ORR 34.9%, CR 19.4% |
| phase I/II | CD20/CD3 | mosunetuzumab | ORR 42%, CR 23.9%; mPFS 3.2 months | |
| phase I/II | CD20/CD3 | subcutaneous mosunetuzumab | ORR 36%, CR 20% | |
| NCT03671018 | phase Ib | CD20/CD3, CD79b | mosunetuzumab-polatuzumab | ORR: ≥65 years 72% vs. <65 years 54%; CR: ≥65 years 56% vs. <65 years 38% |
| NCT04889716 | phase II | CD20/CD3, CD19 | CAR-T followed by mosunetuzumab or glofitamab | NA |
| NCT05672251 | phase II | CD20/CD3, CD19 | mosunetuzumab-loncastuximab | NA |
| NCT05464329 | phase Ib | CD20/CD3 | DHAP or ICE + mosunetuzumab and ASCT | NA |
| NCT03075696 | phase I | CD20/CD3, CD20 | glofitamab-obinutuzumab | ORR 71.4%, CR 64.3% |
| phase II | CD20/CD3, CD20 | glofitamab-obinutuzumab fixed duration | ORR 95%, CR 39%; estimated 12 months OS 50% | |
| NCT04408638 | phase III | CD20/CD3, CD20 | glofitamab-GemOx vs. rituximab-GemOx | NA |
| NCT05364424 | phase Ib | CD20/CD3, CD20 | glofitamab -R-ICE | NA |
| NCT05335018 | phase II | CD20/CD3, BTK | glofitamab-poseltinib-lenalidomide | ORR 100%, CR 50%, PR 50%; mDOR 4 months |
| NCT02290951 | phase I | CD20/CD3 | odronextamab | not CAR-T exposed: ORR and CR 53%; CAR-T exposed: ORR 33%, CR 27%; mDOR NR |
| NCT03888105 | phase II | CD20/CD3 | odronextamab | ORR 53%, CR 37%; mDOR NR |
| NCT02924402 | phase I | CD20/CD3 | plamotamab | ORR 47.4%, CR 26.3% |
| NCT05328102 | phase II | CD20/CD3, CD19 | plamotamab-tafasitamab-lenalidomide | NA |
GDP: gemcitabine-dexamethasone-cisplatin; CAR-T: chimeric antigen receptor-T cells; DHAP: dexamethasone-cytarabine-platinum; ICE: ifosfamide-carboplatin-etoposide; ASCT: autologous stem-cell transplant; GemOx: gemcitabine-oxaliplatin; R-ICE: rituximab-ifosfamide-carboplatin-etoposide; ORR: overall response rate; CR: complete response; mPFS: median progression-free survival; OS: overall survival; mDOR: median duration of response; NA: not available; NR: not reached.
5. Antibody–Drug Conjugates
Antibody–drug conjugates (ADCs) selectively deliver cytotoxins to cancer cells, combining the specificity of mAbs with the cytotoxic effects of chemotherapy drugs. Three elements are fundamental in an ADC structure: the mAb, the covalent linker and the payload. Once attached to its cell-surface antigen, an ADC is internalized, delivering the payload to cancer cells [66]. Cytotoxins may also be released in the extracellular space either from targeted cell apoptosis or monoclonal antibody dissociation, potentially causing bystander non-targeted or antigen-negative tumor cell death, thus improving ADC efficiency [67]. The cytotoxic payloads validated in the clinical setting are categorized into two groups: DNA-interacting agents (e.g., calicheamicin, doxorubicin, pyrrolobenzodiazepine dimer) and tubulin inhibitors (e.g., maytansines and auristatins) [68].
Polatuzumab vedotin is an anti-CD79b mAb conjugated with the microtubule inhibitor monomethyl auristatin E (MMAE). CD79b is involved in B-cell receptor (BCR) signaling and widely expressed in the B-cell lineage [69]. In R/R DLBCL, polatuzumab vedotin showed modest activity as monotherapy (ORR 56%, CR 16%, mPFS 5.0 months, mDOR 5.2 months) [70] and in association with rituximab (ORR 54%, CR 21%, mPFS 5.6 months, mDOR 13.4 months) [71]. The GO29365 study evaluated pola-BR in ASCT-ineligible or ASCT-failure R/R DLBCL. Compared to BR, pola-BR achieved a higher PFS that was improved irrespective of the clinical and biological characteristics [72]. The long-term follow-up data of GO29365 confirmed the persistence of a significantly improved response and survival compared to BR at a median follow-up of approximately 50 months [73]. The most common toxicities include peripheral neuropathy, anemia, neutropenia and thrombocytopenia. Based on the GO29365 study, the FDA and EMA approved polatuzumab vedotin in combination with rituximab and bendamustine in R/R DLBCL who received at least two prior therapies.
Loncastuximab tesirine is a humanized anti-CD19 antibody conjugated with SG3199, a DNA cytotoxic agent [74]. CD19 is a pan-B marker highly expressed throughout B-cell development and maturation, until the stage of plasma cells, when CD19 expression is lost [75]. The LOTIS-2 study investigated loncastuximab tesirine as monotherapy in heavily pretreated R/R DLBCL, showing a significant antitumor activity. The objective response rate and complete response were 48.3% and 24.1%, respectively; the median DOR, PFS and OS were 10.3, 4.9 and 9.9 months [76]. Major AEs emerging during phase 1 dose escalation included neutropenia, thrombocytopenia, edema, liver test abnormalities, hypokalemia and skin/nail toxicity [77]; however, after the introduction of dexamethasone premedication, standard spironolactone diuretics and more stringent recommendations on sun exposure during a phase 2 study, pyrrolobenzodiazepine-related toxicities were generally mild-to-moderate, reversible and manageable [76]. In April 2021, the FDA and EMA approved loncastuximab tesirine for the treatment of R/R large B-cell lymphoma.
Brentuximab vedotin is an anti-CD30 antibody conjugated to monomethyl auristatin E, a microtubule-disrupting agent [78]. CD30 is expressed on a small subset of B and T cells in normal tissues, while its expression is typically high in classical Hodgkin lymphoma (cHL), anaplastic large-cell lymphoma (ALCL), cutaneous T-cell lymphoma (CTCL) and primary mediastinal B-cell lymphoma (PMBCL) [68]. Brentuximab vedotin as monotherapy in R/R DLBCL has been investigated in a phase 2 trial, showing an ORR of 44% (CR 17%, PR 27%) and a median PFS and DOR of 4 and 5.6 months, respectively. No correlation between the CD30 expression level and response was demonstrated, highlighting a plausible bystander killing effect [79]. A phase 1/dose-expansion study assessed the efficacy of brentuximab vedotin in combination with lenalidomide in R/R DLBCL. The ORR was 57% (CR 35%, PR 22%), mPFS 10.2 months and mDOR 13.1 months. Based on these results, a phase 3 study of brentuximab in combination with lenalidomide and rituximab has been initiated (ECHELON-3, NCT04404283) [80]. The most common AEs of brentuximab vedotin include fatigue, gastrointestinal disorders (e.g., diarrhea, nausea), neutropenia, pyrexia and peripheral sensory neuropathy [79].
Pinatuzumab vedotin is an anti-CD22 monoclonal antibody linked to the monomethyl auristatin E (MMAE) [81]. CD22 is involved in BCR signaling and widely expressed in more than 95% B-NHL [82], representing a potential target for the treatment of B-NHL. Pinatuzumab vedotin as monotherapy achieved an ORR of 36% (CR 16%, PR 20%) with a median PFS and DOR of 4.0 and 3.0 months, respectively. The ORR, median PFS and DOR were improved to 60% (CR 26%, PR 33%), 5.4 months and 6.2 months in combination with rituximab, as shown in the ROMULUS study. The most common adverse events included neutropenia, fatigue, peripheral sensory neuropathy, hyperglycemia and anemia [71,81].
Inotuzumab ozogamicin is a humanized anti-CD22 antibody conjugated to calicheamicin. Its antitumor activity has been studied in combination with rituximab, showing encouraging results in R/R DLBCL and an acceptable safety profile. The ORR was 74% (CR 50%), mPFS 17.1 and mDOR 17.7 months. Common toxicities included thrombocytopenia, neutropenia, hyperbilirubinemia and liver test abnormalities [83]. This combination has been investigated as an induction treatment followed by ASCT in R/R DLBCL, with unsatisfactory results [84]. In a phase 1 study, inotuzumab in combination with R-GDP achieved an ORR of 33% (CR 19%) with manageable toxicity, although gemcitabine and cisplatin doses were lower than in the standard R-GDP regimen due to hematologic toxicity [85].
Newly developed ADCs are under investigation in clinical trials of R/R B-NHLs (including DLBCL) (Table 3). Some have failed to achieve significant antitumor activity results, others have demonstrated encouraging results and several others are currently being tested [68].
Table 3.
Antibody–drug conjugates clinical trials.
| NCT | Phase | Targets | Drugs | Results |
|---|---|---|---|---|
| NCT01290549 | phase I | CD79b | polatuzumab vedotin | ORR 56%, CR 16%; mPFS 5.0 months, mDOR 5.2 months |
| NCT01209130 | phase I | CD79b | pinatuzumab vedotin | ORR 36%, CR 16%; mPFS 4.0 months, mDOR 3.0 months |
| NCT01691898 | phase II | CD79b, CD20 | rituximab-polatuzumab | ORR 54%, CR 21%; mPFS 5.6 months, mDOR 13.4 months |
| rituximab-pinatuzumab | ORR 60%, CR 26%; mPFS 5.4 months, mDOR 6.2 months | |||
| NCT02257567 | phase Ib/II | CD79b, CD20 | polatuzumab-BR | ORR 45%, CR 40%; mPFS 9.5 months, mDOR 12.6 months |
| NCT04665765 | phase II | CD79b, CD20 | polatuzumab-R-ICE | NA |
| NCT02611323 | phase Ib/II | CD79b, CD20 | polatuzumab-obinutuzumab-venetoclax | NA |
| polatuzumab-rituximab-venetoclax | NA | |||
| NCT02729896 | phase Ib | CD79b, CD20, PD-L1 | rituximab-atezolizumab-polatuzumab | ORR 25%, CR 13% |
| NCT03589469 | phase II | CD19 | loncastuximab tesirine | ORR 48.3%, CR 24.1%; mPFS 4.9 months, mDOR 10.3 months |
| NCT04384484 | phase III | CD19, CD20 | loncastuximab-rituximab | ORR 75%, CR 40%, PR 35% |
| NCT03685344 | phase I | CD19, PD-L1 | loncastuximab-durvalumab | NA |
| NCT01421667 | phase II | CD30 | brentuximab vedotin | ORR 44%, CR 17%; mPFS 4.0 months, mDOR 5.6 months |
| NCT02086604 | phase I | CD30 | BV-lenalidomide | ORR 57%, CR 35%; mPFS 10.2 months, mDOR 13.1 months |
| NCT04404283 | phase III | CD30, CD20 | rituximab-BV-lenalidomide | ORR 70%, CR 4 patients, PR 2 patients |
| NCT00299494 | phase I/II | CD22, CD20 | rituximab-inotuzumab | ORR 74%, CR 50%; mPFS 17.1 months, mDOR 17.7 months |
BR: bendamustine-rituximab; R-ICE: rituximab-ifosfamide-carboplatin-etoposide; BV: brentuximab vedotin; ORR: overall response rate; CR: complete response; mPFS: median progression-free survival; mDOR: median duration of response; NA: not available.
6. Radioimmunotherapy
Radioimmunotherapy (RIT) consists of a mAb conjugated to a radionuclide that, like ADC, delivers β or γ-particles to targeted tumor cells as well as nearby tumor cells, limiting the dose of radiation delivered to normal tissues. Bystander killing allows for the eradication of tumor cells that are not necessarily targeted by the mAb [86].
Ibritumomab tiuxetan consists of 90yttrium, a pure beta-emitter radioisotope, conjugated to the anti-CD20 ibritumomab. The efficacy of ibritumomab tiuxetan was evaluated in R/R DLBCL in a prospective, multicenter, non-randomized phase 2 study for elderly patients. R/R DLBCL previously treated with a rituximab-based regimen exhibited lower responses and survivals (mPFS 1.6 months, mOS 4.6 months) compared to R/R DLBCL previously treated with chemotherapy only [87]. As expected, responses and survival were higher in patients without prior rituximab exposure compared to rituximab-exposed patients.
Ibritumomab tiuxetan combined with high-dose BEAM (Z-BEAM) and ASCT in R/R DLBCL was demonstrated to be efficient and safe without any additional toxicities compared to BEAM alone (2 years PFS 59% vs 30%), although the PFS difference was not statistically significant (p = 0.2) [88]. A recent meta-analysis showed that patients receiving Z-BEAM have a better OS (84.5%) and PFS (67.2%) at 2 years (p < 0.01 and p < 0.05) than those receiving BEAM. These data need to be further assessed with an appropriate prospective, multicenter and randomized study.
7. Immune Checkpoint Inhibitors
The immune system is frequently impaired in patients with hematological malignancies, both in treatment-naïve and in treated patients. Additionally, cancer cells may develop mutations capable of eluding immune checkpoints [89]. Molecular alterations involved in immunosurveillance escape are frequently observed in DLBCL and include (i) the lack of MHC class I expression (~60% of cases) due to genetic and/or epigenetic lesions in components of the HLA-I complex (e.g., β2-microglobulin, CD58 and HLA-loci); (ii) the downregulation of MHC class II (40–50% of cases), which hampers the presentation of potentially tumor-specific antigens to T cells; (iii) the overexpression of PD-L1 and PD-L2 (~20% of cases) through copy number gain, amplification and chromosomic translocation; and (iv) the increased expression of CD47 on the cancer cell surface [90,91,92]. These alterations cooperate to impair adaptive and innate immune activity against lymphoma. Immune checkpoint inhibitors (ICIs) may restore an appropriate host immune response against lymphomatous cells, potentially inducing a remission of the disease.
Normally, programmed cell death protein 1 (PD-1) and its ligands PD-L1/PD-L2 are essential for maintaining a self-tolerance environment and preventing autoimmunity [93]. However, the overexpression of PD-L1/PD-L2 by cancer cells or nonmalignant cells in the tumor microenvironment, such as tumor-associated macrophages (TAMs), hides malignant cells from the immune system. This immune escape mechanism is frequently observed in lymphomas, like classic Hodgkin lymphoma and primary mediastinal large B-cell lymphoma (PMBCL) [26,94], and to a lesser extent in DLBCL [91]. In fact, the blockade of PD-1 or PD-L1/PD-L2 enhances the immune response against lymphomatous cells. On these bases, several clinical trials have investigated the role of anti-PD-1/PD-L1 mAbs in B-cell malignancies in the relapsed/refractory setting, particularly pembrolizumab and nivolumab, which are anti-PD-1 mAbs, and atezolizumab, an anti-PD-L1 mAb. Although encouraging results have been observed in R/R cHL and R/R PMBCL, R/R DLBCL seem to be resistant to the blockade of PD-1 signaling, achieving only an ORR of 3–10% with nivolumab monotherapy [95]. These results may be partially explained by the low rate of PD-L1 overexpression in DLBCL. Nevertheless, the response to ICIs does not rely merely on the extent of PD-L1 expression, but it is a complex phenomenon involving both tumor and host characteristics. Combination regimens of anti-PD-1/PD-L1 mAbs with novel drugs (e.g., pomalidomide, mosunetuzumab) may achieve higher responses and are currently under investigation in clinical trials (Table 4).
Table 4.
Immune checkpoint inhibitors clinical trials.
| NCT | Phase | Targets | Drugs | Results |
|---|---|---|---|---|
| NCT02038933 | phase II | PD-1 | nivolumab | ORR 3–10%, CR 0–3%; mDOR 8–11.4 months |
| NCT02953509 | phase Ib | CD47, CD20 | magrolimab-rituximab | ORR 40%, CR 33% |
| NCT03527147 | phase I | CD47, CD20, BTK | magrolimab-rituximab-acalabrutinib | ORR 28.6%, CR 28.6% |
| NCT02953509 | phase Ib | CD47, CD20 | magrolimab-rituximab-GemOx | ORR 51.5%, CR 39.4%; mPFS 3.9 months, mDOR 18.0 months |
GemOx: gemcitabine-oxaliplatin; ORR: overall response rate; CR: complete response; mPFS: median progression-free survival; mDOR: median duration of response; NA: not available.
Enhancing the adaptive immune response is not the sole mechanism to be triggered against cancer cells. Macrophages play a critical role in both innate and adaptive immunity. However, their function is frequently subverted in the tumor microenvironment, where they assume a phenotype, called M2, characterized by immunosuppressive and pro-tumorigenic properties [96]. Particularly, the overexpression of CD47 on the cancer cell surface and its interaction with signal regulatory protein α (SIRPα) on macrophages inhibit the phagocytic functions of macrophages through a so-called “do not eat me” signal [97]. Anti-CD47 mAbs block this interaction, revert macrophage phagocytic activity and induce T-cell cytotoxic response via the cross-presentations of tumor antigens on the MHC class I complex [98,99]. In a phase 1b clinical trial, magrolimab, an anti-CD47 mAb, in combination with rituximab achieved an ORR of 40% (CR 33%, PR 7%) among patients with R/R DLBCL [100]. The combination of magrolimab with rituximab and acalabrutinib, a BTK inhibitor, was investigated in a small cohort of R/R DLBCL, achieving an ORR of 28.6% (CR 28.6%) [101]. Higher responses (ORR 51.5%, CR 39.4%; mPFS 3.9 months, mDOR 18.0 months) were achieved in a phase 1b study with the combination of magrolimab and rituximab with chemotherapy [102]. Fatigue, chills, headache, anemia and infusion-related reactions were the most common treatment toxicities, and they were mostly grade 1 or 2 [100].
8. Conclusions
Primary refractory/relapsed DLBCL remains a challenging disease, particularly in those patients who are not eligible for ASCT/CAR-T-cell therapy, failed these treatments or experienced more than one relapse.
The landscape of anti-DLBCL treatment is evolving, and only recently, the replacement of vincristine with polatuzumab vedotin in the R-CHOP—the pola-R-CHP regimen—has demonstrated higher results as a frontline treatment [18]. In recent years, many innovative mAbs and therapeutic approaches have been developed, along with new targets, to overcome chemo-refractoriness and achieve higher response rates in the setting of R/R DLBCL. The role and survival advantages of several of these new mAbs have been well established in various lymphomas, for instance, brentuximab vedotin in cHL, systemic ALCL and in CTCL or pembrolizumab in R/R cHL. However, not all these mAbs are as effective in R/R DLBCL as they are in other lymphoma types, probably due to different biology, high disease heterogeneity, mechanisms of resistance and levels of antigen expression.
Unbound mAbs, bispecific T-cell engagers and antibody–drug conjugates provided a clinical benefit in the setting of R/R DLBCL, while the data regarding radioimmunotherapy and immune checkpoint inhibitors are less promising.
Overall, mAbs have demonstrated a tolerable safety profile. Common adverse events include hematological toxicities such as anemia, neutropenia and thrombocytopenia, as well as infusion-related reactions. Specific adverse events include peripheral neuropathy for ADCs (e.g., brentuximab vedotin, polatuzumab tesirine) and CRS and ICANS for BiTes (e.g., epcoritamab, mosunetuzumab).
Future perspectives in R/R DLBCL treatment with mAbs involve the exploration of novel targets and the development of combination regimens that can simultaneously and synergically engage more pathways, together with a personalized approach based on the molecular characteristics of the disease. Clinical trials are underway to assess the combination of mAbs with other treatments, aiming to further improve outcomes and overcome resistance. Promising results have been achieved in several clinical trials, particularly bispecific T-cell engagers are emerging as an effective and more accessible alternative to CAR-T.
Moreover, lymphomas are not the only hematological malignancies that experienced a significant shift in the treatment landscape due to the impact of mAbs. A notable example is multiple myeloma, where the anti-CD38 daratumumab has become a cornerstone in current induction regimens.
In conclusion, mAbs have represented and still represent a “game changer” of the course of several hematological malignancies, particularly of B-cell lymphomas; starting from rituximab to polatuzumab vedotin, mAbs have boldly achieved results that no other therapy has attained before in the context of lymphoma.
Author Contributions
M.S. and G.M.R.: conceptualization, methodology, investigation, writing—original draft preparation, writing—review and editing, visualization. G.M.-C., A.M.M., W.A.E. and R.B.: writing—review and editing. G.G.: conceptualization, validation, resources, writing—review and editing, visualization, supervision, project administration. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
M.S., R.B., G.M.-C., A.M.M., W.A.E. and G.M.R. have nothing to declare. G.G. declares advisory board and speaker’s bureau honoraria from AbbVie, AstraZeneca, BeiGene, Hikma, Incyte, Janssen and Lilly.
Funding Statement
This work has been supported by Molecular bases of disease dissemination in lymphoid malignancies to optimize curative therapeutic strategies, (5 × 1000 No. 21198), Associazione Italiana per la Ricerca sul Cancro Foundation Milan, Italy; PNRR-MAD-2022–12375673 (Next Generation EU, M6/C2_CALL 2022), Italian Ministry of Health, Rome, Italy; AGING Project—Department of Excellence—DIMET, Università del Piemonte Orientale, Novara, Italy; Ricerca Finalizzata 2018 (project RF-2018-12365790), MoH, Rome, Italy; and AIL Novara Onlus, Novara, Italy.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Li S., Young K.H., Medeiros L.J. Diffuse Large B-Cell Lymphoma. Pathology. 2018;50:74–87. doi: 10.1016/j.pathol.2017.09.006. [DOI] [PubMed] [Google Scholar]
- 2.Perry A.M., Diebold J., Nathwani B.N., Maclennan K.A., Müller-Hermelink H.K., Bast M., Boilesen E., Armitage J.O., Weisenburger D.D. Non-Hodgkin Lymphoma in the Developing World: Review of 4539 Cases from the International Non-Hodgkin Lymphoma Classification Project. Haematologica. 2016;101:1244–1250. doi: 10.3324/haematol.2016.148809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smith A., Crouch S., Lax S., Li J., Painter D., Howell D., Patmore R., Jack A., Roman E. Lymphoma Incidence, Survival and Prevalence 2004–2014: Sub-Type Analyses from the UK’s Haematological Malignancy Research Network. Br. J. Cancer. 2015;112:1575–1584. doi: 10.1038/bjc.2015.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coiffier B., Lepage E., Brière J., Herbrecht R., Tilly H., Bouabdallah R., Morel P., Van Den Neste E., Salles G., Gaulard P., et al. Chop Chemotherapy plus Rituximab Compared with Chop Alone in Elderly Patients with Diffuse Large-B-Cell Lymphoma. N. Engl. J. Med. 2002;346:235–242. doi: 10.1056/NEJMoa011795. [DOI] [PubMed] [Google Scholar]
- 5.Liu Y., Barta S.K. Diffuse Large B-Cell Lymphoma: 2019 Update on Diagnosis, Risk Stratification, and Treatment. Am. J. Hematol. 2019;94:604–616. doi: 10.1002/ajh.25460. [DOI] [PubMed] [Google Scholar]
- 6.Cunningham D., Hawkes E.A., Jack A., Qian W., Smith P., Mouncey P., Pocock C., Ardeshna K.M., Radford J.A., McMillan A., et al. Rituximab plus Cyclophosphamide, Doxorubicin, Vincristine, and Prednisolone in Patients with Newly Diagnosed Diffuse Large B-Cell Non-Hodgkin Lymphoma: A Phase 3 Comparison of Dose Intensification with 14-Day versus 21-Day Cycles. Lancet. 2013;381:1817–1826. doi: 10.1016/S0140-6736(13)60313-X. [DOI] [PubMed] [Google Scholar]
- 7.Delarue R., Tilly H., Mounier N., Petrella T., Salles G., Thieblemont C., Bologna S., Ghesquières H., Hacini M., Fruchart C., et al. Dose-Dense Rituximab-CHOP Compared with Standard Rituximab-CHOP in Elderly Patients with Diffuse Large B-Cell Lymphoma (the LNH03-6B Study): A Randomised Phase 3 Trial. Lancet Oncol. 2013;14:525–533. doi: 10.1016/S1470-2045(13)70122-0. [DOI] [PubMed] [Google Scholar]
- 8.Bartlett N.L., Wilson W.H., Jung S.H., Hsi E.D., Maurer M.J., Pederson L.D., Polley M.Y.C., Pitcher B.N., Cheson B.D., Kahl B.S., et al. Dose-Adjusted EPOCH-R Compared with R-CHOP as Frontline Therapy for Diffuse Large B-Cell Lymphoma: Clinical Outcomes of the Phase III Intergroup Trial Alliance/CALGB 50303. J. Clin. Oncol. 2019;37:1790–1799. doi: 10.1200/JCO.18.01994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sehn L.H., Martelli M., Trněný M., Liu W., Bolen C.R., Knapp A., Sahin D., Sellam G., Vitolo U., Sehn L.H. A Randomized, Open-Label, Phase III Study of Obinutuzumab or Rituximab plus CHOP in Patients with Previously Untreated Diffuse Large B-Cell Lymphoma: Final Analysis of GOYA. J. Hematol. Oncol. 2020;13:71. doi: 10.1186/s13045-020-00900-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jaeger U., Trneny M., Melzer H., Praxmarer M., Nawarawong W., Ben Yehuda D., Goldstein D., Mihaljevic B., Ilhan O., Ballova V., et al. Rituximab Maintenance for Patients with Aggressive B-Cell Lymphoma in First Remission: Results of the Randomized NHL13 Trial. Haematologica. 2015;100:955–963. doi: 10.3324/haematol.2015.125344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thieblemont C., Tilly H., Da Silva M.G., Casasnovas R.O., Fruchart C., Morschhauser F., Haioun C., Lazarovici J., Grosicka A., Perrot A., et al. Lenalidomide Maintenance Compared with Placebo in Responding Elderly Patients with Diffuse Large B-Cell Lymphoma Treated with First-Line Rituximab Plus Cyclophosphamide, Doxorubicin, Vincristine, and Prednisone. J. Clin. Oncol. 2017;35:2473–2481. doi: 10.1200/JCO.2017.72.6984. [DOI] [PubMed] [Google Scholar]
- 12.Witzig T.E., Tobinai K., Rigacci L., Ikeda T., Vanazzi A., Hino M., Shi Y., Mayer J., Costa L.J., Silva C.D.B., et al. Adjuvant Everolimus in High-Risk Diffuse Large B-Cell Lymphoma: Final Results from the PILLAR-2 Randomized Phase III Trial. Ann. Oncol. 2018;29:707–714. doi: 10.1093/annonc/mdx764. [DOI] [PubMed] [Google Scholar]
- 13.Leonard J.P., Kolibaba K.S., Reeves J.A., Tulpule A., Flinn I.W., Kolevska T., Robles R., Flowers C.R., Collins R., DiBella N.J., et al. Randomized Phase II Study of R-CHOP with or without Bortezomib in Previously Untreated Patients with Non-Germinal Center B-Cell-like Diffuse Large B-Cell Lymphoma. J. Clin. Oncol. 2017;35:3538–3546. doi: 10.1200/JCO.2017.73.2784. [DOI] [PubMed] [Google Scholar]
- 14.González-Barca E., Carrillo-Cruz E., Grande C., Martín A., Coronado M., Montes-Moreno S., Mercadal S., Roncero J.M., de Oteyza J.P., Nicolás C., et al. Phase 2 Randomized Trial Comparing Standard RCHOP Versus Brcap (Bortezomib, Rituximab, Cyclophosphamide, Adriamycin and Prednisone) as First Line Treatment in Young Patients with High-Risk Diffuse Large B-Cell Lymphoma (DLBCL). A Study from Spanish Group. Blood. 2016;128:4201. doi: 10.1182/blood.V128.22.4201.4201. [DOI] [Google Scholar]
- 15.Younes A., Sehn L.H., Johnson P., Zinzani P.L., Hong X., Zhu J., Patti C., Belada D., Samoilova O., Suh C., et al. Randomized Phase III Trial of Ibrutinib and Rituximab plus Cyclophosphamide, Doxorubicin, Vincristine, and Prednisone in Non–Germinal Center B-Cell Diffuse Large B-Cell Lymphoma. J. Clin. Oncol. 2019;37:1285–1295. doi: 10.1200/JCO.18.02403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davies A., Cummin T.E., Barrans S., Maishman T., Mamot C., Novak U., Caddy J., Stanton L., Kazmi-Stokes S., McMillan A., et al. Gene-Expression Profiling of Bortezomib Added to Standard Chemoimmunotherapy for Diffuse Large B-Cell Lymphoma (REMoDL-B): An Open-Label, Randomised, Phase 3 Trial. Lancet Oncol. 2019;20:649–662. doi: 10.1016/S1470-2045(18)30935-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nowakowski G.S., Hong F., Scott D.W., Macon W.R., King R.L., Habermann T.M., Wagner-Johnston N., Casulo C., Wade J.L., Nagargoje G.G., et al. Addition of Lenalidomide to R-CHOP Improves Outcomes in Newly Diagnosed Diffuse Large B-Cell Lymphoma in a Randomized Phase II US Intergroup Study ECOG-ACRIN E1412. J. Clin. Oncol. 2021;39:1329–1338. doi: 10.1200/JCO.20.01375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tilly H., Morschhauser F., Sehn L.H., Friedberg J.W., Trněný M., Sharman J.P., Herbaux C., Burke J.M., Matasar M., Rai S., et al. Polatuzumab Vedotin in Previously Untreated Diffuse Large B-Cell Lymphoma. N. Engl. J. Med. 2022;386:351–363. doi: 10.1056/NEJMoa2115304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheson B.D., Pfistner B., Juweid M.E., Gascoyne R.D., Specht L., Horning S.J., Coiffier B., Fisher R.I., Hagenbeek A., Zucca E., et al. Revised Response Criteria for Malignant Lymphoma. J. Clin. Oncol. 2007;25:579–586. doi: 10.1200/JCO.2006.09.2403. [DOI] [PubMed] [Google Scholar]
- 20.Maurer M.J., Ghesquières H., Jais J.P., Witzig T.E., Haioun C., Thompson C.A., Delarue R., Micallef I.N., Peyrade F., Macon W.R., et al. Event-Free Survival at 24 Months Is a Robust End Point for Disease-Related Outcome in Diffuse Large B-Cell Lymphoma Treated with Immunochemotherapy. J. Clin. Oncol. 2014;32:1066–1073. doi: 10.1200/JCO.2013.51.5866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Crump M., Neelapu S.S., Farooq U., Van Den Neste E., Kuruvilla J., Westin J., Link B.K., Hay A., Cerhan J.R., Zhu L., et al. Outcomes in Refractory Diffuse Large B-Cell Lymphoma: Results from the International SCHOLAR-1 Study. Blood. 2017;130:1800–1808. doi: 10.1182/blood-2017-03-769620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gisselbrecht C., Schmitz N., Mounier N., Gill D.S., Linch D.C., Trneny M., Bosly A., Milpied N.J., Radford J., Ketterer N., et al. Rituximab Maintenance Therapy after Autologous Stem-Cell Transplantation in Patients with Relapsed CD20+ Diffuse Large B-Cell Lymphoma: Final Analysis of the Collaborative Trial in Relapsed Aggressive Lymphoma. J. Clin. Oncol. 2012;30:4462–4469. doi: 10.1200/JCO.2012.41.9416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Köhler G., Milstein C. Continuous Cultures of Fused Cells Secreting Antibody of Predefined Specificity. Nature. 1975;256:495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- 24.Buss N.A.P.S., Henderson S.J., McFarlane M., Shenton J.M., De Haan L. Monoclonal Antibody Therapeutics: History and Future. Curr. Opin. Pharmacol. 2012;12:615–622. doi: 10.1016/j.coph.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 25.Goebeler M.E., Bargou R.C. T Cell-Engaging Therapies—BiTEs and Beyond. Nat. Rev. Clin. Oncol. 2020;17:418–434. doi: 10.1038/s41571-020-0347-5. [DOI] [PubMed] [Google Scholar]
- 26.Alsaab H.O., Sau S., Alzhrani R., Tatiparti K., Bhise K., Kashaw S.K., Iyer A.K. PD-1 and PD-L1 Checkpoint Signaling Inhibition for Cancer Immunotherapy: Mechanism, Combinations, and Clinical Outcome. Front. Pharmacol. 2017;8:561. doi: 10.3389/fphar.2017.00561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jurczak W., Zinzani P.L., Gaidano G., Goy A., Provencio M., Nagy Z., Robak T., Maddocks K., Buske C., Ambarkhane S., et al. Phase IIa Study of the CD19 Antibody MOR208 in Patients with Relapsed or Refractory B-Cell Non- Hodgkin’s Lymphoma. Ann. Oncol. 2018;29:1266–1272. doi: 10.1093/annonc/mdy056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Salles G., Duell J., Barca E.G., Tournilhac O., Jurczak W., Liberati A.M., Nagy Z., Obr A., Gaidano G., André M., et al. Tafasitamab plus Lenalidomide in Relapsed or Refractory Diffuse Large B-Cell Lymphoma (L-MIND): A Multicentre, Prospective, Single-Arm, Phase 2 Study. Lancet. Oncol. 2020;21:978–988. doi: 10.1016/S1470-2045(20)30225-4. [DOI] [PubMed] [Google Scholar]
- 29.Woyach J.A., Awan F., Flinn I.W., Berdeja J.G., Wiley E., Mansoor S., Huang Y., Lozanski G., Foster P.A., Byrd J.C. A Phase 1 Trial of the Fc-Engineered CD19 Antibody XmAb5574 (MOR00208) Demonstrates Safety and Preliminary Efficacy in Relapsed CLL. Blood. 2014;124:3553–3560. doi: 10.1182/blood-2014-08-593269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gribben J.G., Fowler N., Morschhauser F. Mechanisms of Action of Lenalidomide in B-Cell Non-Hodgkin Lymphoma. J. Clin. Oncol. 2015;33:2803–2811. doi: 10.1200/JCO.2014.59.5363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sawalha Y. Relapsed/Refractory Diffuse Large B-Cell Lymphoma: A Look at the Approved and Emerging Therapies. J. Pers. Med. 2021;11:1345. doi: 10.3390/jpm11121345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Czuczman M.S., Trněný M., Davies A., Rule S., Linton K.M., Wagner-Johnston N., Gascoyne R.D., Slack G.W., Brousset P., Eberhard D.A., et al. A Phase 2/3 Multicenter, Randomized, Open-Label Study to Compare the Efficacy and Safety of Lenalidomide versus Investigator’s Choice in Patients with Relapsed or Refractory Diffuse Large B-Cell Lymphoma. Clin. Cancer Res. 2017;23:4127–4137. doi: 10.1158/1078-0432.CCR-16-2818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang M., Fowler N., Wagner-Bartak N., Feng L., Romaguera J., Neelapu S.S., Hagemeister F., Fanale M., Oki Y., Pro B., et al. Oral Lenalidomide with Rituximab in Relapsed or Refractory Diffuse Large Cell, Follicular and Transformed Lymphoma: A Phase II Clinical Trial. Leukemia. 2013;27:1902–1909. doi: 10.1038/leu.2013.95. [DOI] [PubMed] [Google Scholar]
- 34.Wiernik P.H., Lossos I.S., Tuscano J.M., Justice G., Vose J.M., Cole C.E., Lam W., McBride K., Wride K., Pietronigro D., et al. Lenalidomide Monotherapy in Relapsed or Refractory Aggressive Non-Hodgkin’s Lymphoma. J. Clin. Oncol. 2008;26:4952–4957. doi: 10.1200/JCO.2007.15.3429. [DOI] [PubMed] [Google Scholar]
- 35.Duell J., Maddocks K.J., González-Barca E., Jurczak W., Liberati A.M., de Vos S., Nagy Z., Obr A., Gaidano G., Abrisqueta P., et al. Long-Term Outcomes from the Phase II L-MIND Study of Tafasitamab (MOR208) plus Lenalidomide in Patients with Relapsed or Refractory Diffuse Large B-Cell Lymphoma. Haematologica. 2021;106:2417–2426. doi: 10.3324/haematol.2020.275958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Duell J., Jurczak W., Liberati A.M., Halka J., Carbó E.P., Costa P.A., Maddocks K.J., Dreyling M., Rosenwald A., Bakuli A., et al. L-Mind: A Safety and Efficacy Analysis of Tafasitamab in Patients with Relapsed/Refractory Diffuse Large B-Cell Lymphoma (R/R DLBCL) Receiving Treatment for at Least 2 Years. Blood. 2022;140:6596–6598. doi: 10.1182/blood-2022-159983. [DOI] [Google Scholar]
- 37.Larsen T.S., Manzke O., Leibovitz D., Arbushites M. A Phase 3 Study of Tafasitamab Plus Lenalidomide in Patients with Relapsed or Refractory Diffuse Large B-Cell Lymphoma (FirmMIND) Blood. 2022;140:12077–12078. doi: 10.1182/blood-2022-160319. [DOI] [Google Scholar]
- 38.Nowakowski G.S., Yoon D.H., Mondello P., Joffe E., Peters A., Fleury I., Greil R., Ku M., Marks R., Kim K., et al. RE-MIND2: Comparative Effectiveness of Tafasitamab plus Lenalidomide versus Polatuzumab Vedotin/Bendamustine/Rituximab (Pola-BR), CAR-T Therapies, and Lenalidomide/Rituximab (R2) Based on Real-World Data in Patients with Relapsed/Refractory Diffuse Large. Ann. Hematol. 2023;102:1773–1787. doi: 10.1007/s00277-023-05196-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cordoba R., Prawitz T., Westley T., Sharma A., Ambarkhane S., Kapetanakis V., Sabatelli L. Tafasitamab Plus Lenalidomide Versus 3 Rituximab-Based Treatments for Non-Transplant Eligible Relapsed/Refractory Diffuse Large B-Cell Lymphoma: A Matching-Adjusted Indirect Comparison. Adv. Ther. 2022;39:2668–2687. doi: 10.1007/s12325-022-02094-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nowakowski G.S., Duell J., Kopeckova K., Trneny M., Pott C., Khouja M., Burke J.M., Waldron-Lynch M., Wagner S., Mukhopadhyay A., et al. First-Mind: Final Analysis from a Phase Ib, Open-Label, Randomized Study to Assess Safety of Tafasitamab or Tafasitamab + Lenalidomide in Addition to R-CHOP in Patients with Newly Diagnosed Diffuse Large B-Cell Lymphoma. Blood. 2022;140:3731–3733. doi: 10.1182/blood-2022-163160. [DOI] [Google Scholar]
- 41.Vitolo U., Nowakowski G.S., Burke J.M., Fox C.P., Trneny M., Chiappella A., Waldron-Lynch M., Wagner S., Pachori A., Lenz G. Frontmind: A Phase III, Multicenter, Randomized, Double-Blind Study of Tafasitamab + Lenalidomide + R-CHOP Versus R-CHOP Alone for Newly Diagnosed High-Intermediate and High-Risk Diffuse Large B-Cell Lymphoma. Blood. 2022;140:6618–6620. doi: 10.1182/blood-2022-155995. [DOI] [Google Scholar]
- 42.Engelberts P.J., Hiemstra I.H., de Jong B., Schuurhuis D.H., Meesters J., Hernandez I.B., Oostindie S.C., Neijssen J., van den Brink E.N., Horbach G.J., et al. DuoBody-CD3xCD20 Induces Potent T-Cell-Mediated Killing of Malignant B Cells in Preclinical Models and Provides Opportunities for Subcutaneous Dosing. EBioMedicine. 2020;52:102625. doi: 10.1016/j.ebiom.2019.102625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van der Horst H.J., de Jonge A.V., Hiemstra I.H., Gelderloos A.T., Berry D.R.A.I., Hijmering N.J., van Essen H.F., de Jong D., Chamuleau M.E.D., Zweegman S., et al. Epcoritamab Induces Potent Anti-Tumor Activity against Malignant B-Cells from Patients with DLBCL, FL and MCL, Irrespective of Prior CD20 Monoclonal Antibody Treatment. Blood Cancer J. 2021;11:38. doi: 10.1038/s41408-021-00430-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hutchings M., Mous R., Clausen M.R., Johnson P., Linton K.M., Chamuleau M.E.D., Lewis D.J., Balari A.S., Cunningham D., Oliveri R.S., et al. Dose Escalation of Subcutaneous Epcoritamab in Patients with Relapsed or Refractory B-Cell Non-Hodgkin Lymphoma: An Open-Label, Phase 1/2 Study. Lancet. 2021;398:1157–1169. doi: 10.1016/S0140-6736(21)00889-8. [DOI] [PubMed] [Google Scholar]
- 45.Thieblemont C., Phillips T., Ghesquieres H., Cheah C.Y., Clausen M.R., Cunningham D., Do Y.R., Feldman T., Gasiorowski R., Jurczak W., et al. Epcoritamab, a Novel, Subcutaneous CD3 × CD20 Bispecific T-Cell-Engaging Antibody, in Relapsed or Refractory Large B-Cell Lymphoma: Dose Expansion in a Phase I/II Trial. J. Clin. Oncol. 2023;41:2238–2247. doi: 10.1200/JCO.22.01725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Abrisqueta P., Cordoba R., Falchi L., de Vos S., Nijland M., Offner F., Wu J., Bykhovski I., Wang L., Rana A., et al. Subcutaneous Epcoritamab + R-Dhax/C in Patients with Relapsed or Refractory Diffuse Large B-Cell Lymphoma Eligible for Autologous Stem Cell Transplant: Updated Phase 1/2 Results. Blood. 2022;140:1068–1069. doi: 10.1182/blood-2022-158278. [DOI] [Google Scholar]
- 47.Budde L.E., Assouline S., Sehn L.H., Schuster S.J., Yoon S.-S., Yoon D.H., Matasar M.J., Bosch F., Kim W.S., Nastoupil L.J., et al. Single-Agent Mosunetuzumab Shows Durable Complete Responses in Patients with Relapsed or Refractory B-Cell Lymphomas: Phase I Dose-Escalation Study. J. Clin. Oncol. 2021;40:481–491. doi: 10.1200/JCO.21.00931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schuster S.J., Bartlett N.L., Assouline S., Yoon S.-S., Bosch F., Sehn L.H., Cheah C.Y., Shadman M., Gregory G.P., Ku M., et al. Mosunetuzumab Induces Complete Remissions in Poor Prognosis Non-Hodgkin Lymphoma Patients, Including Those Who Are Resistant to or Relapsing After Chimeric Antigen Receptor T-Cell (CAR-T) Therapies, and Is Active in Treatment through Multiple Lines. Blood. 2019;134:6. doi: 10.1182/blood-2019-123742. [DOI] [Google Scholar]
- 49.Bartlett N.L., Giri P., Budde L.E., Schuster S.J., Assouline S., Matasar M.J., Yoon S.-S., Canales M., Gutierrez N.C., Fay K., et al. Subcutaneous (SC) Administration of Mosunetuzumab with Cycle 1 Step-up Dosing Is Tolerable and Active in Patients with Relapsed/Refractory B-Cell Non-Hodgkin Lymphomas (R/R B-NHL): Initial Results from a Phase I/II Study. Blood. 2021;138:3573. doi: 10.1182/blood-2021-147937. [DOI] [Google Scholar]
- 50.Matasar M.J., Cheah C.Y., Yoon D.H., Assouline S.E., Bartlett N.L., Ku M., Giri P., Johnston A., Flinn I.W., Goy A.H., et al. Subcutaneous Mosunetuzumab in Relapsed or Refractory B-Cell Lymphoma: Promising Safety and Encouraging Efficacy in Dose Escalation Cohorts. Blood. 2020;136:45–46. doi: 10.1182/blood-2020-135818. [DOI] [Google Scholar]
- 51.Budde E.L., Bartlett N.L., Giri P., Schuster S.J., Assouline S., Yoon S.-S., Fay K., Matasar M.J., Gutierrez N.C., Marlton P., et al. Subcutaneous Mosunetuzumab Is Active with a Manageable Safety Profile in Patients (Pts) with Relapsed/Refractory (R/R) B-Cell Non-Hodgkin Lymphomas (B-NHLs): Updated Results from a Phase I/II Study. Blood. 2022;140:3753–3755. doi: 10.1182/blood-2022-157729. [DOI] [Google Scholar]
- 52.Olszewski A.J., Budde L.E., Chavez J., Ghosh N., Kamdar M., Lossos I.S., Diefenbach C., Sabry W., Dorritie K., Huw L.-Y., et al. Mosunetuzumab with Polatuzumab Vedotin Is Effective and Has a Manageable Safety Profile in Patients Aged <65 and ≥65 Years with Relapsed/Refractory Diffuse Large B-Cell Lymphoma (R/R DLBCL) and ≥1 Prior Therapy: Subgroup Analysis of a Phase Ib/II Study. Blood. 2022;140:3757–3759. doi: 10.1182/blood-2022-159594. [DOI] [Google Scholar]
- 53.Bacac M., Colombetti S., Herter S., Sam J., Perro M., Chen S., Bianchi R., Richard M., Schoenle A., Nicolini V., et al. CD20-TCB with Obinutuzumab Pretreatment as next-Generation Treatment of Hematologic Malignancies. Clin. Cancer Res. 2018;24:4785–4797. doi: 10.1158/1078-0432.CCR-18-0455. [DOI] [PubMed] [Google Scholar]
- 54.Hutchings M., Morschhauser F., Iacoboni G., Carlo-Stella C., Offner F.C., Sureda A., Salles G., Martínez-Lopez J., Crump M., Thomas D.N., et al. Glofitamab, a Novel, Bivalent CD20-Targeting T-Cell-Engaging Bispecific Antibody, Induces Durable Complete Remissions in Relapsed or Refractory B-Cell Lymphoma: A Phase I Trial. J. Clin. Oncol. 2021;39:1959–1970. doi: 10.1200/JCO.20.03175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dickinson M.J., Carlo-Stella C., Morschhauser F., Bachy E., Corradini P., Iacoboni G., Khan C., Wróbel T., Offner F., Trněný M., et al. Glofitamab for Relapsed or Refractory Diffuse Large B-Cell Lymphoma. N. Engl. J. Med. 2022;387:2220–2231. doi: 10.1056/NEJMoa2206913. [DOI] [PubMed] [Google Scholar]
- 56.Ferhanoglu B., Gulbas Z., Uzay A., Özcan M., Ozkalemkas F., Dal M.S., Kalyon H., Akay O.M., Deveci B., Bekoz H., et al. Glofitamab in Relapsed/Refractory Diffuse Large B Cell Lymphoma: Real World Data. Blood. 2022;140:6704–6705. doi: 10.1182/blood-2022-165385. [DOI] [Google Scholar]
- 57.Hutchings M., Carlo-Stella C., Morschhauser F., Bachy E., Corradini P., Iacoboni G., Khan C., Patel K., Hertzberg M., Falchi L., et al. Relapse Is Uncommon in Patients with Large B-Cell Lymphoma Who Are in Complete Remission at the End of Fixed-Course Glofitamab Treatment. Blood. 2022;140:1062–1064. doi: 10.1182/blood-2022-157554. [DOI] [Google Scholar]
- 58.Hutchings M., Carlo-Stella C., Gritti G., Bosch F., Morschhauser F., Townsend W., Offner F., Walter H.S., Ghesquieres H., Houot R., et al. CD19 4-1BBL (RO7227166) a Novel Costimulatory Bispecific Antibody Can Be Safely Combined with the T-Cell-Engaging Bispecific Antibody Glofitamab in Relapsed or Refractory B-Cell Non-Hodgkin Lymphoma. Blood. 2022;140:9461–9463. doi: 10.1182/blood-2022-157011. [DOI] [Google Scholar]
- 59.Topp M.S., Tani M., Dickinson M., Ghosh N., Santoro A., Pinto A., Bosch F., Fox C.P., López-Guillermo A., Carlucci C., et al. Glofitamab Plus R-CHOP Induces High Response Rates and a Favorable Safety Profile in Patients with Previously Untreated Diffuse Large B-Cell Lymphoma (DLBCL): Results from a Phase Ib Study. Blood. 2022;140:1775–1777. doi: 10.1182/blood-2022-157732. [DOI] [Google Scholar]
- 60.Smith E.J., Olson K., Haber L.J., Varghese B., Duramad P., Tustian A.D., Oyejide A., Kirshner J.R., Canova L., Menon J., et al. A Novel, Native-Format Bispecific Antibody Triggering T-Cell Killing of B-Cells Is Robustly Active in Mouse Tumor Models and Cynomolgus Monkeys. Sci. Rep. 2015;5:17943. doi: 10.1038/srep17943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bannerji R., Arnason J.E., Advani R.H., Brown J.R., Allan J.N., Ansell S.M., Barnes J.A., O’Brien S.M., Chávez J.C., Duell J., et al. Odronextamab, a Human CD20 × CD3 Bispecific Antibody in Patients with CD20-Positive B-Cell Malignancies (ELM-1): Results from the Relapsed or Refractory Non-Hodgkin Lymphoma Cohort in a Single-Arm, Multicentre, Phase 1 Trial. Lancet Haematol. 2022;9:e327–e339. doi: 10.1016/S2352-3026(22)00072-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kim W.-S., Kim T.M., Cho S.-G., Jarque I., Iskierka-Jażdżewska E., Poon M.L., Prince H.M., Oh S.Y., Lim F., Carpio C., et al. Odronextamab in Patients with Relapsed/Refractory (R/R) Diffuse Large B-Cell Lymphoma (DLBCL): Results from a Prespecified Analysis of the Pivotal Phase II Study ELM-2. Blood. 2022;140:1070–1071. doi: 10.1182/blood-2022-158406. [DOI] [Google Scholar]
- 63.Wei J., Montalvo-Ortiz W., Yu L., Krasco A., Olson K., Rizvi S., Fiaschi N., Coetzee S., Wang F., Ullman E., et al. CD22-Targeted CD28 Bispecific Antibody Enhances Antitumor Efficacy of Odronextamab in Refractory Diffuse Large B Cell Lymphoma Models. Sci. Transl. Med. 2022;14:eabn1082. doi: 10.1126/scitranslmed.abn1082. [DOI] [PubMed] [Google Scholar]
- 64.Patel K., Michot J.-M., Chanan-Khan A.A., Salles G.A., Cartron G., Peyrade F., Bouabdallah R., Reid E.G., Thomas S.K., Wierda W.G., et al. Preliminary Safety and Anti-Tumor Activity of XmAb13676, an Anti-CD20 × Anti-CD3 Bispecific Antibody, in Patients with Relapsed/Refractory Non-Hodgkin’s Lymphoma and Chronic Lymphocytic Leukemia. Blood. 2019;134:4079. doi: 10.1182/blood-2019-128564. [DOI] [Google Scholar]
- 65.Patel K., Riedell P.A., Tilly H., Ahmed S., Michot J.-M., Ghesquieres H., de Collela J.M.S., Chanan-Khan A., Bouabdallah K., Tessoulin B., et al. A Phase 1 Study of Plamotamab, an Anti-CD20 × Anti-CD3 Bispecific Antibody, in Patients with Relapsed/Refractory Non-Hodgkin’s Lymphoma: Recommended Dose Safety/Efficacy Update and Escalation Exposure-Response Analysis. Blood. 2022;140:9470–9472. doi: 10.1182/blood-2022-159586. [DOI] [Google Scholar]
- 66.Thomas A., Teicher B.A., Hassan R. Antibody–Drug Conjugates for Cancer Therapy. Lancet. Oncol. 2016;17:e254. doi: 10.1016/S1470-2045(16)30030-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Staudacher A.H., Brown M.P. Antibody Drug Conjugates and Bystander Killing: Is Antigen-Dependent Internalisation Required? Br. J. Cancer. 2017;117:1736–1742. doi: 10.1038/bjc.2017.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chu Y., Zhou X., Wang X. Antibody-Drug Conjugates for the Treatment of Lymphoma: Clinical Advances and Latest Progress. J. Hematol. Oncol. 2021;14:88. doi: 10.1186/s13045-021-01097-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dornan D., Bennett F., Chen Y., Dennis M., Eaton D., Elkins K., French D., Go M.A.T., Jack A., Junutula J.R., et al. Therapeutic Potential of an Anti-CD79b Antibody–Drug Conjugate, Anti–CD79b-vc-MMAE, for the Treatment of Non-Hodgkin Lymphoma. Blood. 2009;114:2721–2729. doi: 10.1182/blood-2009-02-205500. [DOI] [PubMed] [Google Scholar]
- 70.Palanca-Wessels M.C.A., Czuczman M., Salles G., Assouline S., Sehn L.H., Flinn I., Patel M.R., Sangha R., Hagenbeek A., Advani R., et al. Safety and Activity of the Anti-CD79B Antibody–Drug Conjugate Polatuzumab Vedotin in Relapsed or Refractory B-Cell Non-Hodgkin Lymphoma and Chronic Lymphocytic Leukaemia: A Phase 1 Study. Lancet Oncol. 2015;16:704–715. doi: 10.1016/S1470-2045(15)70128-2. [DOI] [PubMed] [Google Scholar]
- 71.Morschhauser F., Flinn I.W., Advani R., Sehn L.H., Diefenbach C., Kolibaba K., Press O.W., Salles G., Tilly H., Chen A.I., et al. Polatuzumab Vedotin or Pinatuzumab Vedotin plus Rituximab in Patients with Relapsed or Refractory Non-Hodgkin Lymphoma: Final Results from a Phase 2 Randomised Study (ROMULUS) Lancet Haematol. 2019;5:e254–e265. doi: 10.1016/S2352-3026(19)30026-2. [DOI] [PubMed] [Google Scholar]
- 72.Sehn L.H., Herrera A.F., Flowers C.R., Kamdar M.K., McMillan A., Hertzberg M., Assouline S., Kim T.M., Kim W.S., Ozcan M., et al. Polatuzumab Vedotin in Relapsed or Refractory Diffuse Large B-Cell Lymphoma. J. Clin. Oncol. 2020;38:155–165. doi: 10.1200/JCO.19.00172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sehn L.H., Hertzberg M., Opat S., Herrera A.F., Assouline S., Flowers C.R., Kim T.M., McMillan A., Ozcan M., Safar V., et al. Polatuzumab Vedotin plus Bendamustine and Rituximab in Relapsed/Refractory DLBCL: Survival Update and New Extension Cohort Data. Blood Adv. 2022;6:533–543. doi: 10.1182/bloodadvances.2021005794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zammarchi F., Corbett S., Adams L., Tyrer P.C., Kiakos K., Janghra N., Marafioti T., Britten C.E., Havenith C.E.G., Chivers S., et al. ADCT-402, a PBD Dimer–Containing Antibody Drug Conjugate Targeting CD19-Expressing Malignancies. Blood. 2018;131:1094–1105. doi: 10.1182/blood-2017-10-813493. [DOI] [PubMed] [Google Scholar]
- 75.Wang K., Wei G., Liu D. CD19: A Biomarker for B Cell Development, Lymphoma Diagnosis and Therapy. Exp. Hematol. Oncol. 2012 11. 2012;1:36. doi: 10.1186/2162-3619-1-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Caimi P.F., Ai W., Alderuccio J.P., Ardeshna K.M., Hamadani M., Hess B., Kahl B.S., Radford J., Solh M., Stathis A., et al. Loncastuximab Tesirine in Relapsed or Refractory Diffuse Large B-Cell Lymphoma (LOTIS-2): A Multicentre, Open-Label, Single-Arm, Phase 2 Trial. Lancet Oncol. 2021;22:790–800. doi: 10.1016/S1470-2045(21)00139-X. [DOI] [PubMed] [Google Scholar]
- 77.Hamadani M., Radford J., Carlo-Stella C., Caimi P.F., Reid E., O’Connor O.A., Feingold J.M., Ardeshna K.M., Townsend W., Solh M., et al. Final Results of a Phase 1 Study of Loncastuximab Tesirine in Relapsed/Refractory B-Cell Non-Hodgkin Lymphoma. Blood. 2021;137:2634–2645. doi: 10.1182/blood.2020007512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Connors J.M., Jurczak W., Straus D.J., Ansell S.M., Kim W.S., Gallamini A., Younes A., Alekseev S., Illés Á., Picardi M., et al. Brentuximab Vedotin with Chemotherapy for Stage III or IV Hodgkin’s Lymphoma. N. Engl. J. Med. 2018;378:331–344. doi: 10.1056/NEJMoa1708984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jacobsen E.D., Sharman J.P., Oki Y., Advani R.H., Winter J.N., Bello C.M., Spitzer G., Palanca-Wessels M.C., Kennedy D.A., Levine P., et al. Brentuximab Vedotin Demonstrates Objective Responses in a Phase 2 Study of Relapsed/Refractory DLBCL with Variable CD30 Expression. Blood. 2015;125:1394–1402. doi: 10.1182/blood-2014-09-598763. [DOI] [PubMed] [Google Scholar]
- 80.Ward J.P., Berrien-Elliott M.M., Gomez F., Luo J., Becker-Hapak M., Cashen A.F., Wagner-Johnston N.D., Maddocks K., Mosior M., Foster M., et al. Phase 1/Dose Expansion Trial of Brentuximab Vedotin and Lenalidomide in Relapsed or Refractory Diffuse Large B-Cell Lymphoma. Blood. 2022;139:1999–2010. doi: 10.1182/blood.2021011894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Advani R.H., Lebovic D., Chen A., Brunvand M., Goy A., Chang J.E., Hochberg E., Yalamanchili S., Kahn R., Lu D., et al. Phase i Study of the Anti-CD22 Antibody-Drug Conjugate Pinatuzumab Vedotin with/without Rituximab in Patients with Relapsed/Refractory B-Cell Non-Hodgkin Lymphoma. Clin. Cancer Res. 2017;23:1167–1176. doi: 10.1158/1078-0432.CCR-16-0772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Walker J.A., Smith K.G.C. CD22: An Inhibitory Enigma. Immunology. 2008;123:314–325. doi: 10.1111/j.1365-2567.2007.02752.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fayad L., Offner F., Smith M.R., Verhoef G., Johnson P., Kaufman J.L., Rohatiner A., Advani A., Foran J., Hess G., et al. Safety and Clinical Activity of a Combination Therapy Comprising Two Antibody-Based Targeting Agents for the Treatment of Non-Hodgkin Lymphoma: Results of a Phase I/II Study Evaluating the Immunoconjugate Inotuzumab Ozogamicin with Rituximab. J. Clin. Oncol. 2013;31:573–583. doi: 10.1200/JCO.2012.42.7211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wagner-Johnston N.D., Goy A., Rodriguez M.A., Ehmann W.C., Hamlin P.A., Radford J., Thieblemont C., Suh C., Sweetenham J., Huang Y., et al. A Phase 2 Study of Inotuzumab Ozogamicin and Rituximab, Followed by Autologous Stem Cell Transplant in Patients with Relapsed/Refractory Diffuse Large B-Cell Lymphoma. Leuk. Lymphoma. 2015;56:2863–2869. doi: 10.3109/10428194.2015.1017821. [DOI] [PubMed] [Google Scholar]
- 85.Sangha R., Davies A., Dang N.H., Ogura M., MacDonald D.A., Ananthakrishnan R., Paccagnella M.L., Vandendries E., Boni J., Goh Y.T. Phase 1 Study of Inotuzumab Ozogamicin Combined with R-GDP for the Treatment of Patients with Relapsed/Refractory CD22+ B-Cell Non-Hodgkin Lymphoma. J. Drug Assess. 2017;6:10. doi: 10.1080/21556660.2017.1315336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rizzieri D. Zevalin® (Ibritumomab Tiuxetan): After More than a Decade of Treatment Experience, What Have We Learned? Crit. Rev. Oncol. Hematol. 2016;105:5–17. doi: 10.1016/j.critrevonc.2016.07.008. [DOI] [PubMed] [Google Scholar]
- 87.Morschhauser F., Illidge T., Huglo D., Martinelli G., Paganelli G., Zinzani P.L., Rule S., Liberati A.M., Milpied N., Hess G., et al. Efficacy and Safety of Yttrium-90 Ibritumomab Tiuxetan in Patients with Relapsed or Refractory Diffuse Large B-Cell Lymphoma Not Appropriate for Autologous Stem-Cell Transplantation. Blood. 2007;110:54–58. doi: 10.1182/blood-2007-01-068056. [DOI] [PubMed] [Google Scholar]
- 88.Shimoni A., Avivi I., Rowe J.M., Yeshurun M., Levi I., Or R., Patachenko P., Avigdor A., Zwas T., Nagler A. A Randomized Study Comparing Yttrium-90 Ibritumomab Tiuxetan (Zevalin) and High-Dose BEAM Chemotherapy versus BEAM Alone as the Conditioning Regimen before Autologous Stem Cell Transplantation in Patients with Aggressive Lymphoma. Cancer. 2012;118:4706–4714. doi: 10.1002/cncr.27418. [DOI] [PubMed] [Google Scholar]
- 89.Kline J., Godfrey J., Ansell S.M. The Immune Landscape and Response to Immune Checkpoint Blockade Therapy in Lymphoma. Blood. 2020;135:523–533. doi: 10.1182/blood.2019000847. [DOI] [PubMed] [Google Scholar]
- 90.Pasqualucci L., Dalla-Favera R. Genetics of Diffuse Large B-Cell Lymphoma. Blood. 2018;131:2307–2319. doi: 10.1182/blood-2017-11-764332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Georgiou K., Chen L., Berglund M., Ren W., De Miranda N.F.C.C., Lisboa S., Fangazio M., Zhu S., Hou Y., Wu K., et al. Genetic Basis of PD-L1 Overexpression in Diffuse Large B-Cell Lymphomas. Blood. 2016;127:3026–3034. doi: 10.1182/blood-2015-12-686550. [DOI] [PubMed] [Google Scholar]
- 92.Chao M.P., Alizadeh A.A., Tang C., Myklebust J.H., Varghese B., Gill S., Jan M., Cha A.C., Chan C.K., Tan B.T., et al. Anti-CD47 Antibody Synergizes with Rituximab to Promote Phagocytosis and Eradicate Non-Hodgkin Lymphoma. Cell. 2010;142:699–713. doi: 10.1016/j.cell.2010.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Freeman G.J., Long A.J., Iwai Y., Bourque K., Chernova T., Nishimura H., Fitz L.J., Malenkovich N., Okazaki T., Byrne M.C., et al. Engagement of the PD-1 Immunoinhibitory Receptor by a Novel B7 Family Member Leads to Negative Regulation of Lymphocyte Activation. J. Exp. Med. 2000;192:1027–1034. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Twa D.D.W., Chan F.C., Ben-Neriah S., Woolcock B.W., Mottok A., Tan K.L., Slack G.W., Gunawardana J., Lim R.S., McPherson A.W., et al. Genomic Rearrangements Involving Programmed Death Ligands Are Recurrent in Primary Mediastinal Large B-Cell Lymphoma. Blood. 2014;123:2062–2065. doi: 10.1182/blood-2013-10-535443. [DOI] [PubMed] [Google Scholar]
- 95.Ansell S.M., Minnema M.C., Johnson P., Timmerman J.M., Armand P., Shipp M.A., Rodig S.J., Ligon A.H., Roemer M.G.M., Reddy N., et al. Nivolumab for Relapsed/Refractory Diffuse Large B-Cell Lymphoma in Patients Ineligible for or Having Failed Autologous Transplantation: A Single-Arm, Phase II Study. J. Clin. Oncol. 2019;37:481–489. doi: 10.1200/JCO.18.00766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Qian B.Z., Pollard J.W. Macrophage Diversity Enhances Tumor Progression and Metastasis. Cell. 2010;141:39–51. doi: 10.1016/j.cell.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tsai R.K., Discher D.E. Inhibition of “Self” Engulfment through Deactivation of Myosin-II at the Phagocytic Synapse between Human Cells. J. Cell Biol. 2008;180:989–1003. doi: 10.1083/jcb.200708043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Liu J., Wang L., Zhao F., Tseng S., Narayanan C., Shura L., Willingham S., Howard M., Prohaska S., Volkmer J., et al. Pre-Clinical Development of a Humanized Anti-CD47 Antibody with Anti-Cancer Therapeutic Potential. PLoS ONE. 2015;10:e0137345. doi: 10.1371/journal.pone.0137345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tseng D., Volkmer J.P., Willingham S.B., Contreras-Trujillo H., Fathman J.W., Fernhoff N.B., Seita J., Inlay M.A., Weiskopf K., Miyanishi M., et al. Anti-CD47 Antibody-Mediated Phagocytosis of Cancer by Macrophages Primes an Effective Antitumor T-Cell Response. Proc. Natl. Acad. Sci. USA. 2013;110:11103–11108. doi: 10.1073/pnas.1305569110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Advani R., Flinn I., Popplewell L., Forero A., Bartlett N.L., Ghosh N., Kline J., Roschewski M., LaCasce A., Collins G.P., et al. CD47 Blockade by Hu5F9-G4 and Rituximab in Non-Hodgkin’s Lymphoma. N. Engl. J. Med. 2018;379:1711–1721. doi: 10.1056/NEJMoa1807315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.de Vos S., Reagan P.M., Patel M.R., Saba N.S., Mortlock A., Cerec V., Munugalavadla V., Acar M., Nuttall B., Jenkins D., et al. Magrolimab, Rituximab and Acalabrutinib for Relapsed/Refractory (R/R) Diffuse Large B-Cell Lymphoma (DLBCL): Results from the Phase 1 PRISM Trial. Blood. 2022;140:6635–6637. doi: 10.1182/blood-2022-162447. [DOI] [Google Scholar]
- 102.Maakaron J., Asch A.S., Popplewell L.L., Collins G.P., Flinn I.W., Ghosh N., Keane C., Ku M., Mehta A., Roschewski M., et al. Magrolimab in Combination with Rituximab + Chemotherapy in Patients with Relapsed or Refractory (R/R) Diffuse Large B-Cell Lymphoma (DLBCL) Blood. 2022;140:3728–3730. doi: 10.1182/blood-2022-167772. [DOI] [Google Scholar]