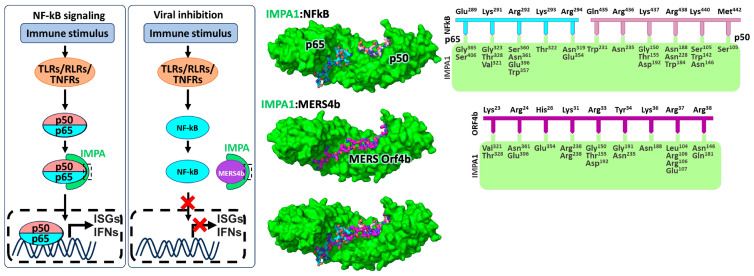
Figure 4.
Interactions and Structural Insights into MERS-CoV ORF4b Inhibition of Immune Signaling. Normally, an immune stimulus would trigger the NF-B pathway, leading to the transport of p50/p65 into the nucleus. The complex of IMPA2 with p50/p65, which facilitates this process, has been structurally characterized (PDB 7LET). The MERS-CoV ORF4b protein can disrupt this interaction, thereby preventing the nuclear import of p50/p65 and suppressing the expression of ISGs and IFNs. Structural analysis has provided insights into the MERS-CoV ORF4b mode of action by revealing a mutually exclusive binding mechanism at the interface, as shown in the structural data from PDB 7RFZ [44]. In the visualization, IMPA is rendered in green with a surface representation, while p50 and p65 are depicted in stick form in salmon and light blue, respectively. These models are constructed based on high-resolution X-ray crystallography data.