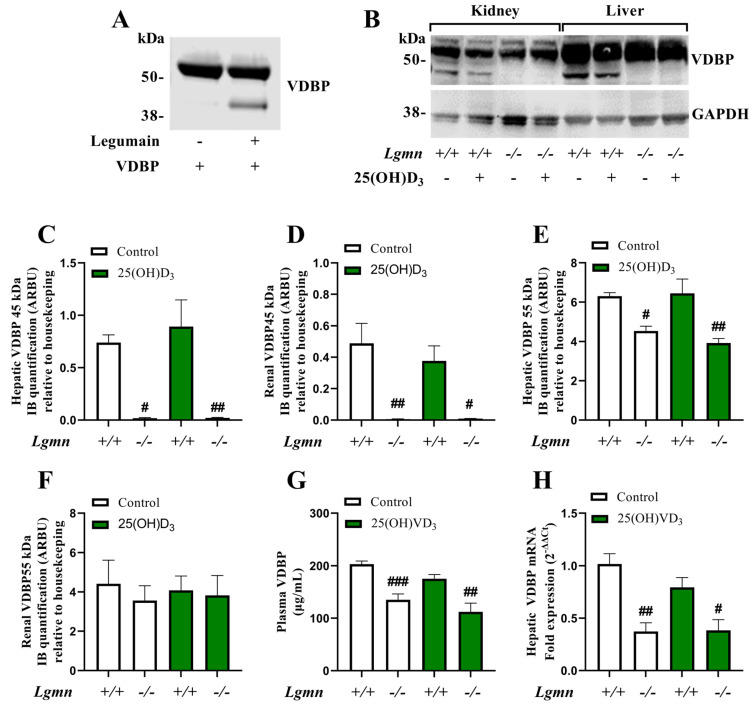
Figure 3.
Legumain is required for VDBP processing and regulation. (A) Purified VDBP from human plasma (1.9 μM) was incubated in legumain assay buffer (pH 5.8) at 37 °C with or without purified active bovine legumain (2 μM) for 5 h before gel electrophoresis and immunoblotting of VDBP (n = 1). (B–H) Wild-type (Lgmn+/+) and legumain-deficient (Lgmn−/−) mice were treated with 50 µg/kg 25(OH)D3 (n = 6–7) or an equal volume vehicle (n = 7, control) subcutaneously every two to three days (four times in total). Tissues were harvested 24 h after the final injection (day 8). (B) One representative immunoblot of VDBP and GAPDH (housekeeping) in kidney and liver (n = 4). (C–F) Quantification of VDBP immunoband (IB) intensity as arbitrary units (ARBU) relative to GAPDH in immunoblots represented in (B) (n = 4). (C) Hepatic VDBP 45 kDa immunoband. (D) Renal VDBP 45 kDa immunoband. (E) Hepatic VDBP 55 kDa immunoband. (F) Renal VDBP 55 kDa immunoband. (G) Plasma VDBP concentration (μg/mL) was measured by ELISA (n = 6–7). (H) Hepatic VDBP mRNA expression relative to the geometric mean of CT values of four housekeeping controls (2−ΔΔCT, n = 5). (C–H) Data represent mean ± SEM. Two-way ANOVA. # p < 0.05, ## p < 0.01, ### p < 0.001 vs. different genotype, same treatment. Numbers (n) represent individual biological replicates.