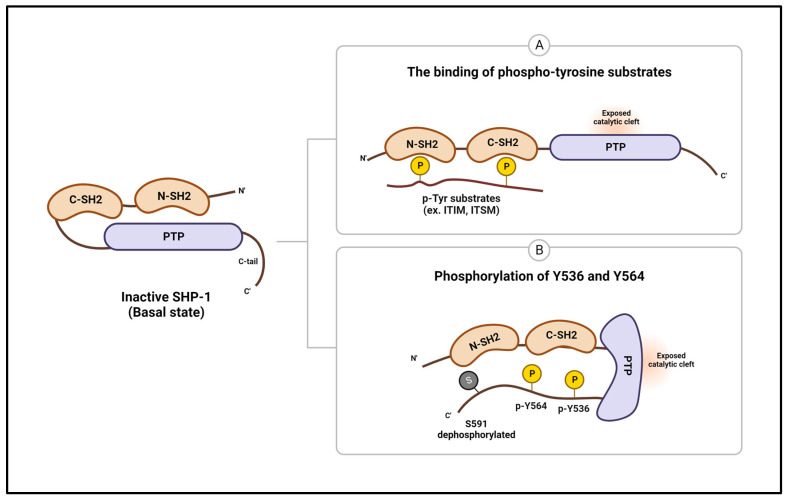
Figure 2.
Protein structures and regulation of SHP-1. The enzymatic activity of SHP-1 is inhibited by intramolecular interaction between the N-SH2 and PTP domains. (A) The binding of the SH2 domain by tyrosine-phosphorylated substrates and (B) phosphorylation of tyrosine residues in the C-terminal tail causes a conformational change that opens the phosphatase active site and contributes to phosphatase activation. ITIM—immunoreceptor tyrosine-based inhibitory motif; ITSM—immunoreceptor tyrosine-based switch motif.