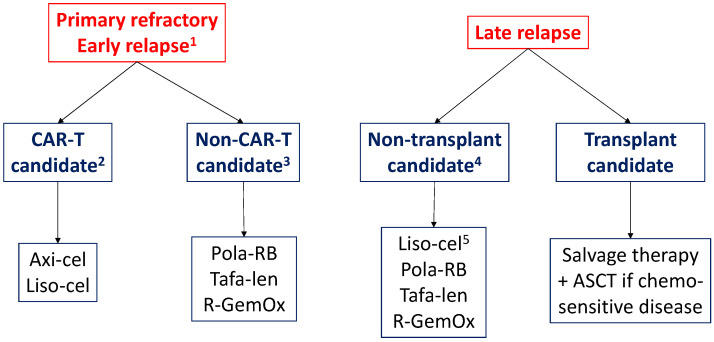
Figure 1.
Proposed algorithm for the second-line treatment of DLBCL. ASCT, autologous stem cell transplantation; axi-cel, axicabtagene ciloleucel; DLBCL, diffuse large B-cell lymphoma; liso-cel, lisocabtagene maraleucel; pola-BR, polatuzumab-vedotin with bendamustine and rituximab; R-GemOx, rituximab, gemcitabine, and oxaliplatin; tafa-len, tafasitamab and lenalidomide. 1 During the first year following the completion of first-line treatment. 2 The selection of treatment (axi-cel or liso-cel) must be individualized based on CAR-T availability and the patient’s characteristics. Liso-cel could be favored in older patients or patients with organ dysfunctions [41]. 3 The selection of treatment must be individualized. In countries where CAR-T cell therapy is available in the third, but not the second line, the administration of bendamustine should be avoided in these high-risk patients due to its prolonged lymphodepletive effect [62]. Tafasitamab should also be avoided because it targets CD19, and the potential implications for CAR-T cell therapy if the patient loses CD19, or its expression density decreases, has not been thoroughly studied [71]. For these patients, GemOx +/− R may be a suitable option. The three regimens (pola-RB, tafa-len and R-GemOx) are more efficacious in relapsed than in primary refractory patients. Its inclusion in clinical trials is especially recommended for the latter group of patients. 4 The selection of treatment must be individualized according to patient’s characteristics and the toxicity profile of each agent or regimen. 5 Liso-cel is approved by the FDA, but not by the EMA, for this indication.