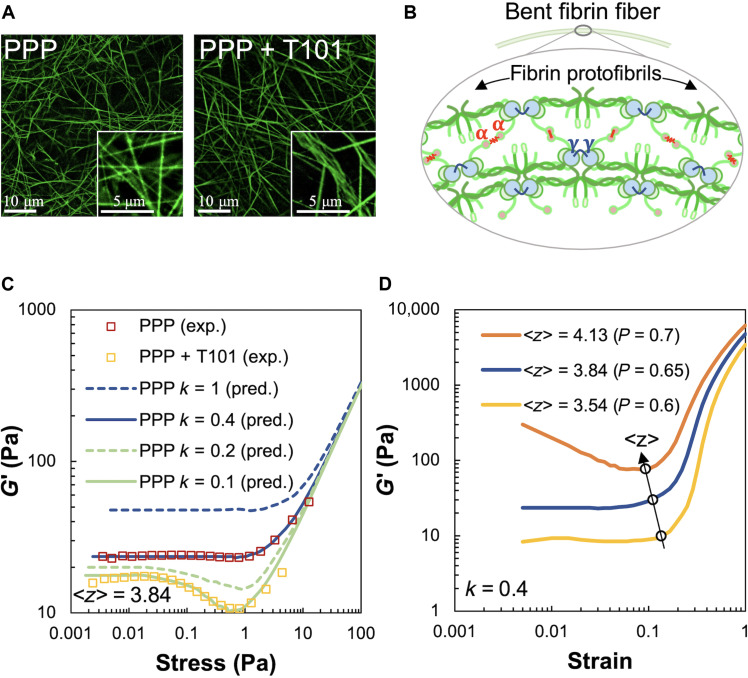
Fig. 2. Effect of fibrin cross-linking on shear stiffness regimes of passive PPP clots.
(A) Microscopy images of cross-linked (PPP) and uncross-linked (PPP + T101) clots. Inset on the right shows loosely cross-linked and crimped protofibrils due to inhibition of FXIII-A by T101 treatment. Scale bars, 10 μm (5 μm for insets). (B) Schematic of bent fibrin fiber showing protofibril structure. Fibrin oligomers assemble into protofibrils, which, in turn, are cross-linked by γ-γ, α-α, and α-γ interactions by FXIII-A. The cross-links are expected to enhance bending stiffness of fibers. (C) Shear modulus (G′) of cross-linked (PPP) and uncross-linked (PPP + T101) clots show a highly nonlinear dependence on applied stress in both experiments (squares, four donors) and simulations (lines). The factor k and ⟨z⟩ in the simulated curves represent the reduced bending modulus from a reference value and average coordination number of network nodes, respectively. Simulations were performed at varying values of the parameters k and ⟨z⟩ to match the experimental data. The model results, at a reduced bending modulus (k = 0.1, solid green line), capture the effect of cross-linker inhibition by T101 treatment on the measured shear modulus (experiments, yellow squares). Both experimental data and model prediction in this case exhibit a noticeable softening dip at ~0.2 Pa. (D) Increasing the average coordination number ⟨z⟩, realized in simulations through less removal of random bonds (p denotes probability of bonds being present), leads to higher shear moduli due to greater number of bond springs and concomitantly reduces the critical strain at which the network abruptly transitions to a stiffer regime. Thus, the position of the transition point in strain can be used to determine the ⟨z⟩ appropriate for the experimental data.