Abstract
Acyl coenzyme A (CoA) synthetase (EC 6.2.1.8) from Pseudomonas fragi catalyzes the synthesis of adenosine 5′-tetraphosphate (p4A) and adenosine 5′-pentaphosphate (p5A) from ATP and tri- or tetrapolyphosphate, respectively. dATP, adenosine-5′-O-[γ-thiotriphosphate] (ATPγS), adenosine(5′)tetraphospho(5′)adenosine (Ap4A), and adenosine(5′)pentaphospho(5′)adenosine (Ap5A) are also substrates of the reaction yielding p4(d)A in the presence of tripolyphosphate (P3). UTP, CTP, and AMP are not substrates of the reaction. The Km values for ATP and P3 are 0.015 and 1.3 mM, respectively. Maximum velocity was obtained in the presence of MgCl2 or CoCl2 equimolecular with the sum of ATP and P3. The relative rates of synthesis of p4A with divalent cations were Mg = Co > Mn = Zn >> Ca. In the pH range used, maximum and minimum activities were measured at pH values of 5.5 and 8.2, respectively; the opposite was observed for the synthesis of palmitoyl-CoA, with maximum activity in the alkaline range. The relative rates of synthesis of palmitoyl-CoA and p4A are around 10 (at pH 5.5) and around 200 (at pH 8.2). The synthesis of p4A is inhibited by CoA, and the inhibitory effect of CoA can be counteracted by fatty acids. To a lesser extent, the enzyme catalyzes the synthesis also of Ap4A (from ATP), Ap5A (from p4A), and adenosine(5′)tetraphospho(5′)nucleoside (Ap4N) from adequate adenylyl donors (ATP, ATPγS, or octanoyl-AMP) and adequate adenylyl acceptors (nucleoside triphosphates).
Dinucleoside polyphosphates have been detected in a wide variety of eukaryotic and prokaryotic organisms (13). In higher organisms, their concentrations are generally on the order of 0.01 to 1 μM. Human blood platelets and chromaffin cells of bovine adrenal medulla contain diadenosine polyphosphates located in the dense bodies (10, 26, 35) and chromaffin granules (32, 38), respectively, where they may reach higher local concentrations. The occurrence of dinucleoside polyphosphates has been described for lower eukaryotic (Saccharomyces cerevisiae, Dictyostelium discoideum, and Physarum polycephalum) and for prokaryotic (Salmonella typhimurium, Escherichia coli, and Clostridium acetobutylicum) organisms (13).
Dinucleoside tetraphosphates participate in the control of purine nucleotide metabolism (36), where Ap4A is an activator of both the IMP-GMP-specific cytosolic 5′-nucleotidase (EC 3.1.3.5) and AMP deaminase (EC 3.5.4.6) (Ka, micromolar range) and Gp4G is an activator of GMP reductase (EC 1.6.6.8) (Ka, nanomolar range) (36). As the concentration of dinucleoside polyphosphates increases under unfavorable environmental conditions, they have been implicated in the cellular response to stress (31). A role of Ap4A in DNA synthesis has been proposed elsewhere (14). Dinucleoside polyphosphates are also transition state analogs of some kinases (37). More recently, the dinucleoside triphosphatase activity of a putative tumor suppressor gene product has been described (3).
The nucleoside 5′-polyphosphates (pnN) are another family of related compounds, p4A has been detected in rabbit and horse muscle (41), rat liver (44), S. cerevisiae spores (19), and chromaffin granules (38). As p4A is a very strong inhibitor (Ki, nanomolar range) of asymmetrical dinucleoside tetraphosphatase (EC 3.6.1.17) (22), changes in the level of p4A could affect the concentration and physiological roles of Ap4A. Other enzymes known to be inhibited (Ki, micromolar range) by p4N are guanylate cyclase (EC 4.6.1.2) (p4A and p4G) (18) and phosphodiesterase I (EC 3.1.4.1) (p4G) (9). Effects of p4A on the tone of the vascular system, mediated by P2 receptors, have also been described elsewhere (21).
The cellular level of dinucleoside polyphosphates results from their rate of degradation and synthesis. The following specific enzymes, implicated in the cleavage of dinucleoside polyphosphates, have been described (see reference 15 for a review): asymmetrical dinucleoside tetraphosphatase (EC 3.6.1.17), symmetrical dinucleoside tetraphosphatase (EC 3.6.1.41), dinucleoside tetraphosphate phosphorylase (EC 2.7.7.53), and dinucleoside triphosphatase (EC 3.6.1.29). In addition, there are other unspecific enzymes able to catalyze the hydrolysis of dinucleoside polyphosphates like E. coli 5′-nucleotidase (34) and phosphodiesterase I (9, 15, 26).
This paper deals with the synthesis of (di)nucleoside polyphosphates. It has been known since 1966 that some aminoacyl tRNA synthetases (30, 45) catalyze the synthesis of Ap4A through reactions 1 and 2:
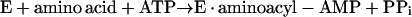 |
reaction 1 |
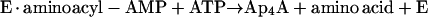 |
reaction 2 |
The possibility that other enzymes (mainly synthetases and some transferases) which catalyze the formation of AMP, via nucleotidyl-containing intermediates and by releasing PPi, could catalyze the synthesis of dinucleoside polyphosphates was later raised (17). Luciferase (EC 1.13.12.7), considered as an oxidoreductase, catalyzes the synthesis of Ap4A with ATP as substrate and luciferin as an essential activator (27, 40):
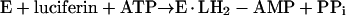 |
reaction 3 |
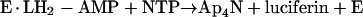 |
reaction 4 |
Acetyl-CoA synthetase (EC 6.2.1.1) from S. cerevisiae also catalyzes the synthesis of p4A and p5A, from ATP and P3 and P4, respectively (16). In the reactions catalyzed by luciferase and acetyl-CoA synthetase, ATP is a very good substrate for the formation of the E · X-AMP complex (X = the appropriate acyl residue), whereas any NTP (or even P3) is an acceptor (particularly in the case of luciferase) of the AMP moiety of the complex, provided that it has an intact terminal pyrophosphate (27, 40).
Here we show that acyl-CoA synthetase from Pseudomonas fragi catalyzes the synthesis of p4A, p5A, Ap4A, Ap5A, and a variety of Ap4Ns. In our view, these findings widen the knowledge of the mechanisms of synthesis of (di)nucleoside polyphosphates in prokaryotes and, by extrapolation, also in eukaryotes.
MATERIALS AND METHODS
Abbreviations.
The abbreviations used are as follows: p4A, adenosine 5′-tetraphosphate; p5A, adenosine 5′-pentaphosphate; p4G, guanosine 5′-tetraphosphate; p4N, nucleoside 5′-tetraphosphate; P3, tripolyphosphate; P4, tetrapolyphosphate; Ap2A, adenosine(5′)diphospho(5′)adenosine; Ap3A, adenosine(5′)triphospho(5′)adenosine; Ap4A, adenosine(5′)tetraphospho(5′)adenosine; Ap5A, adenosine(5′)pentaphospho(5′)adenosine; Ap4N, adenosine(5′)tetraphospho(5′)nucleoside; Ap4C, adenosine(5′)tetraphospho(5′)cytosine; Ap4dC, adenosine(5′)tetraphospho(5′)deoxycytosine; Ap4G, adenosine(5′)tetraphospho(5′)guanosine; Ap4dG, adenosine(5′)tetraphospho(5′)deoxyguanosine; Ap4X, adenosine(5′)tetraphospho(5′)xanthosine; Ap4U, adenosine(5′)tetraphospho(5′)uridine; Ap4dT, adenosine(5′)tetraphospho(5′)thymidine; Gp4G, guanosine(5′)tetraphospho(5′)guanosine; NTP, nucleoside 5′-triphosphate; ATPγS, adenosine 5′-O-[γ-thiotriphosphate]; MES, 2-(N-morpholino)ethanosulfonic acid; CoA, coenzyme A; octanoyl-AMP, octanoyl-adenylate; LH2-AMP, luciferyl-adenylate; U, micromoles of product formed per minute; HPLC, high-performance liquid chromatography; TLC, thin-layer chromatography.
Materials.
Acyl-CoA synthetase from P. fragi was obtained from Boehringer Mannheim. The lyophilized powder was, unless otherwise indicated, dissolved (3.62 mg/ml) in 25 mM HEPES-KOH (pH 7.6)–0.1 mM dithiothreitol–5% glycerol–0.1% bovine serum albumin (solution E) (16). CoA, dithiothreitol, octanoic anhydride, palmitic and octanoic acids, sodium tripolyphosphate, ammonium tetrapolyphosphate, and the nucleotides were from Sigma or Boehringer Mannheim, except for dTTP (Pharmacia Biotech). Bovine serum albumin (fraction V, fatty acid free) was from Boehringer Mannheim. [2,8-3H]ATP (45 Ci/mmol) was from Amersham Life Sciences, and [α-32P]ATP was from DuPont NEN. The stock solutions of 1 mM octanoic acid and 1 mM palmitic acid were prepared by adding enough KOH so that the pH was 7.5; in the case of palmitic acid, the emulsion was further dispersed in 1% Triton X-100 or in 1% Triton X-100–5% ethanol. Phosphodiesterase from Crotalus durissus (EC 3.1.4.1), alkaline phosphatase (EC 3.1.3.1) from calf intestine, and inorganic pyrophosphatase (EC 3.6.1.1) from yeast were purchased from Boehringer Mannheim. Asymmetrical dinucleoside tetraphosphatase was purified from rat liver as described by Sillero et al. (39). Octanoyl-AMP was prepared from AMP and octanoic anhydride as previously described (43). HPLC chromatographs were from Hewlett-Packard or Waters. Ultrafiltration was performed with microconcentrators with exclusion limit membranes of 30 kDa (from Vivascience or Amicon Inc.).
Enzyme assays.
All the assays were carried out at 30°C.
Synthesis of p4A.
Unless otherwise indicated, the reaction mixtures (50 μl) contained 50 mM MES-KOH (pH 6.3), 0.1 mM dithiothreitol, 1 mM ATP, 10 mM P3, 11 mM MgCl2, and acyl-CoA synthetase (5 to 10 μg of protein). The reaction mixtures were analyzed by one of the following methods.
(i) TLC.
Aliquots (3 to 4 μl) of the reaction mixtures were spotted on silica gel plates (TLC UV254 fluorescent chromatographic plates; Merck) and developed in dioxane-ammonium hydroxide-water (6:1:6, by volume). When [2,8-3H]ATP was used, the nucleotide spots, localized with 253-nm-wavelength light, were cut and the radioactivity was counted. When [α-32P]ATP was used, the TLC plates were directly analyzed in an InstantImager (Packard Instrument Co.).
(ii) HPLC.
Aliquots of 10 to 20 μl of the reaction mixtures were diluted in water, kept at 100°C for 90 s, chilled, filtered, and analyzed with Hypersil octyldecyl silane columns (Hewlett-Packard). Elutions were performed at a constant flow rate (0.5 ml/min) with a 20-min linear gradient (5 to 30 mM) of sodium phosphate (pH 7.5) in 20 mM tetrabutylammonium bromide–20% methanol (buffer A), followed by a 10-min linear gradient (30 to 100 mM) of sodium phosphate (pH 7.5) in buffer A or isocratic buffer (15 or 25 mM sodium phosphate, pH 7.5, in buffer A).
Synthesis of palmitoyl-CoA.
Unless otherwise indicated, the reaction mixtures (50 μl) contained 50 mM Tris-HCl (pH 8.2), 0.1 mM dithiothreitol, 1 mM MgCl2, 1 mM [2,8-3H]ATP, 1 mM CoA, 15 μl of the palmitic acid stock solution, and acyl-CoA synthetase (0.27 μg of protein). The analysis of the assay mixtures was carried out as described above, and the plates were developed in dioxane-ammonium hydroxide-water (6:1:5). Activity was measured as the amount of AMP formed.
Synthesis of Ap4A and Ap5A.
The reaction mixtures (0.6 ml) contained 50 mM MES-KOH (pH 5.5), 0.1 mM dithiothreitol, 4 mM MgCl2, 4 mM ATP or p4A, inorganic pyrophosphatase (1.5 μl), and acyl-CoA synthetase (89 μg of protein).
Synthesis of Ap4G or Ap4C from ATP and GTP or CTP.
The reaction mixtures (0.1 ml) contained 50 mM MES-KOH (pH 6.3), 0.1 mM dithiothreitol, 0.63 mM ATP, 3 mM GTP or CTP, 6 mM MgCl2, desalted inorganic pyrophosphatase (1.7 μl), and acyl-CoA synthetase (25 μg of protein).
Miscellaneous methods.
Inorganic pyrophosphatase used to hydrolyze the PPi produced during the enzyme assays or the PPi contaminating P3 was a suspension in ammonium sulfate solution (3.2 M). As the ammonium salts inhibited the synthesis of p4A by acyl-CoA synthetase (unpublished results from this laboratory), pyrophosphatase was desalted by ultrafiltration before use.
HPLC gel filtration of acyl-CoA synthetase was carried out by injecting into a Bio-Sil-Sec 250 column (600 by 7.5 mm; Bio-Rad) 0.5 ml of 1.8 mg of lyophilized powder dissolved in 50 mM Na2SO4–20 mM sodium phosphate (pH 6.8) buffer. Elution was performed at a constant flow rate (0.5 ml/min) with the same buffer. Fractions of 0.25 ml were collected, and p4A and palmitoyl-CoA synthetic activities were measured.
Contaminant ATP was removed from the acyl-CoA synthetase by dialysis for about 30 h at 4°C. In the first 18 h, 1 ml of the enzyme preparation was dialyzed against 1 liter of solution E without albumin replaced by 200 ml of solution E in the last 12 h.
Purification of the mono- or dinucleoside polyphosphates synthesized by acyl-CoA synthetase was performed by TLC. The entire reaction mixtures were heated at 100°C for 90 s (in the case of dinucleoside polyphosphates, the samples were previously treated with alkaline phosphatase [10 μg of protein] for 2 h) and filtered, and the total volume was spotted on silica gel plates along a line and developed in dioxane-ammonium hydroxide-water as described above. The visible (under 253-nm-wavelength light) line spots corresponding to the nucleotides were cut, concentrated by elution with dioxane-water (1:1), and finally extracted with water.
RESULTS
Synthesis of adenosine 5′-polyphosphates (p4A and p5A) from ATP and Pn.
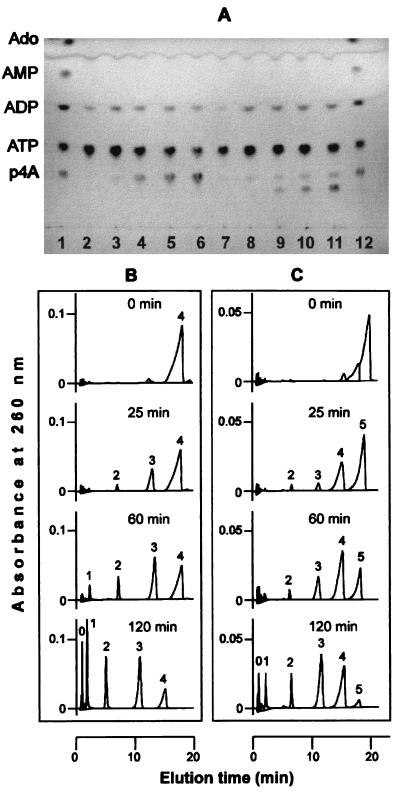
A reaction mixture containing acyl-CoA synthetase, ATP, MgCl2, inorganic pyrophosphatase, and P3 or P4 accumulated compounds with chromatographic (TLC and HPLC) mobilities similar to that of p4A or p5A, respectively. The synthesis of these compounds depended on the presence of enzyme, and their concentration increased with the time of incubation (Fig. 1A). The identity of the corresponding chromatographic peaks was assessed as p4A and p5A by the following criteria: coelution with standards in TLC and HPLC, absorption spectra, and treatment with alkaline phosphatase. This treatment yielded ATP, ADP, AMP, and adenosine in the case of p4A and the same products plus p4A in the case of p5A (Fig. 1B and C).
FIG. 1.
(A) Synthesis of p4A and p5A from ATP, P3, and P4 catalyzed by acyl-CoA synthetase. The reaction mixtures contained 1.15 mM ATP, 0.8 μl of desalted inorganic pyrophosphatase, 5 mM P3 (lanes 2 to 6) or P4 (lanes 7 to 11), and acyl-CoA synthetase (4.9 μg of protein when the polyphosphate added was P3 or 9.8 μg of protein when it was P4); other conditions and TLC analysis procedures were as described in Materials and Methods. Lanes: 1 and 12, standards of p4A, ATP, ADP, AMP, and adenosine; lanes 2 and 7, the control mixtures without acyl-CoA synthetase after 2 and 4 h of incubation, respectively; lanes 3 to 6, the complete mixture containing P3 taken after 0, 0.5, 1, and 2 h of incubation, respectively; lanes 8 to 11, the complete mixture containing P4 taken after 0, 1, 2, and 4 h of incubation, respectively. (B and C) Effect of alkaline phosphatase on the presumptive p4A (B) and p5A (C) synthesized. Similar reaction mixtures (0.8 ml) were incubated for 7 or 36 h (in the case of P3 or P4 as adenylyl acceptor substrate, respectively), and the presumptive p4A or p5A formed was purified (see Materials and Methods) and characterized as follows: reaction mixtures (1 ml) containing 50 mM MES-KOH (pH 6.7), 0.2 mM MgCl2, and purified p4A (100 μM) or p5A (60 μM) were treated with alkaline phosphatase (0.5 μg of protein); at the times indicated, aliquots were taken and analyzed by HPLC. The numbers 0 to 5 on the top of the chromatographic peaks correspond to adenosine, AMP, ADP, ATP, p4A, and p5A, respectively.
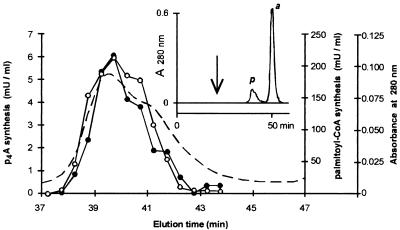
Synthesis of p4A and palmitoyl-CoA by the commercial enzyme preparations. (i) Nucleotide content.
The aim of these experiments was to test whether the synthesis of p4A was catalyzed by the acyl-CoA synthetase or a contaminating enzyme(s) present in the commercial preparations. The thermal inactivation profiles (heating the enzyme preparation at 65°C for 0 to 60 min, followed by cooling on ice) of the activities of synthesis of p4A and palmitoyl-CoA (measured as AMP formed) were coincident, both decreasing to half of those of the nonheated enzyme preparation after 5 min at 65°C (data not shown). The commercial enzyme preparation yielded two peaks (p and a) upon elution from a Bio-Sil-Sec column (Fig. 2). Activities of synthesis of p4A and palmitoyl-CoA coeluted exclusively with peak p, which also had a UV maximum at 280 nm. Peak a had a UV maximum similar to that of adenosine. As some of the experiments performed here (for example, determination of the Km value for ATP) required knowing the nucleotide content of the commercial preparations, five lots of acyl-CoA synthetase were analyzed by HPLC, with a Hypersil octyldecyl silane column. In the enzyme preparations, the concentration of ATP varied between a maximum of 0.56 and a minimum of 0.22 μmol/mg of lyophilized powder; p4A, ADP, and AMP were always less than 0.005, 0.16, and 0.03 μmol/mg, respectively, and no Ap4A or other nucleotide was detected.
FIG. 2.
Coelution of p4A and palmitoyl-CoA synthetic activities upon gel filtration. A sample of acyl-CoA synthetase was applied to a Bio-Sil-Sec 250 column as described in Materials and Methods (inset); the arrow marks the column void volume; peaks p and a correspond to protein and adenine nucleotides, respectively. The activities of synthesis of p4A (•) and palmitoyl-CoA (○) were studied with 15 and 0.33 μl of the column fractions, respectively; [2,8-3H]ATP was used as radioactive substrate. Other conditions were as described in Materials and Methods. The broken line represents absorbance at 280 nm.
(ii) Metal requirement.
The synthesis of p4A depended on the presence of a divalent cation. Maximum activity was obtained in the presence of MgCl2 equimolar with the sum of ATP and P3. Similar profiles were attained with MgCl2 and CoCl2; less activity was measured in the presence of MnCl2 or ZnCl2, whereas with CaCl2, the activity was even lower (results not shown).
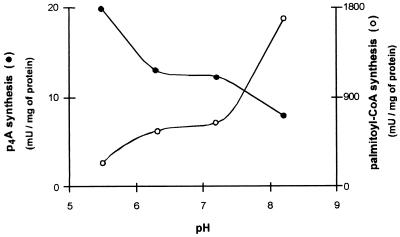
(iii) Effect of pH.
The reaction mixtures for the synthesis of p4A were carried out in 50 mM (each) buffers specified in Fig. 3. The maximal rate was observed at pH 5.5, and at higher pH values, the activity decreased rather steadily. At pH 8.2, the activity was still 40% of that attained at pH 5.5. The activity of synthesis of palmitoyl-CoA, measured with the same buffers and pH range values, was maximal at pH 8.2 and minimal at pH 5.5 (Fig. 3). The relative rates of synthesis of palmitoyl-CoA and p4A are ca. 10 (at pH 5.5) and ca. 200 (at pH 8.2).
FIG. 3.
Effect of pH on the synthesis of p4A and palmitoyl-CoA catalyzed by acyl-CoA synthetase. The reaction mixtures (50 μl) contained 50 mM MES-KOH (pH 5.5 and 6.3), HEPES-KOH (pH 7.2), or Tris-HCl (pH 8.2) and [2,8-3H]ATP as radioactive substrate. In the case of p4A synthesis, 8.3 μg of protein was used; in the case of palmitoyl-CoA synthesis, the enzyme amount varied between 2.0 (pH 5.5) and 0.4 (pH 8.2) μg of protein; other conditions were as described in Materials and Methods.
Two opposite pH profiles for the same enzyme catalyzing two different reactions were also reported in the case of acetyl-CoA synthetase (synthesis of p4A and acetyl-CoA) and for luciferase (synthesis of Ap4A and light production), with optimum pH values in the acid and alkaline range, respectively (12, 16, 23, 40).
(iv) Km values in the synthesis of p4A.
The Km value for P3 in the synthesis of p4A was determined in the presence of fixed (1 mM) ATP, fixed (1 mM) free Mg2+, and variable (1 to 10 mM) P3 concentrations. In these conditions, the Km value found for P3 was 1.3 mM (results not shown). A Km value of 15 μM for ATP was determined in the presence of 10 mM P3 and 10.2 mM MgCl2 (results not shown).
(v) Nucleotide specificity.
The substrate specificity for the synthesis of p4N was studied at pH 5.5 (50 mM MES-KOH) in the presence of fixed concentrations of P3 (10 mM), nucleotide (1 mM), MgCl2 (11 mM), and inorganic pyrophosphatase (0.4% [vol/vol]) and with an enzyme preparation from which ATP had been removed by dialysis (see Materials and Methods). The following nucleotides were assayed as substrates: ATP, ATPγS, dATP, Ap4A, Ap5A, GTP, UTP, CTP, and AMP. The concentration of acyl-CoA synthetase in the assay containing ATP or ATPγS was 42 μg of protein/ml and was six times higher in the assays containing other nucleotides. The reaction mixtures and appropriate controls without enzyme were analyzed by HPLC after 0, 1, 6, and 24 h of incubation. CTP, UTP, and AMP were not substrates. With the other (di)nucleotide tested, enzyme-dependent synthesis of products with chromatographic mobilities and spectra compatible with p4N was observed, the relative enzyme activities being as follows: ATP (100), ATPγS (75), dATP (55), Ap5A (30), Ap4A (15), and GTP (6).
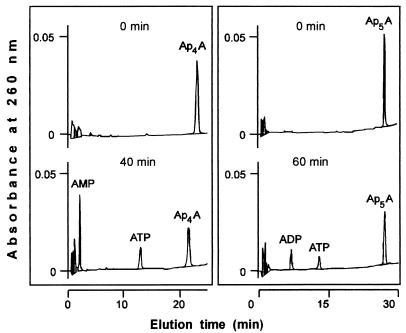
(vi) Synthesis of diadenosine polyphosphates from ATP or p4A.
Acyl-CoA synthetase-dependent synthesis of Ap4A or Ap5A was observed in reaction mixtures containing ATP or p4A, respectively (see Materials and Methods). The relative activities of synthesis of p4A versus Ap4A and p4A versus Ap5A were around 100 and 40, respectively. The synthesized diadenosine polyphosphates were purified and then characterized by treatment with phosphodiesterase from Crotalus durissus or with asymmetrical dinucleoside tetraphosphatase from rat liver. Upon phosphodiesterase treatment, formation of AMP and ATP from Ap4A and of AMP and p4A from Ap5A was firstly observed (results not shown). Upon dinucleoside tetraphosphatase treatment, the products of hydrolysis of Ap4A were AMP and ATP and those from Ap5A were ADP and ATP (Fig. 4). These results, together with the UV spectra and coelution on TLC and HPLC with the corresponding standards, unequivocally characterized the two compounds synthesized by acyl-CoA synthetase as Ap4A and Ap5A, respectively.
FIG. 4.
Effect of asymmetrical dinucleoside tetraphosphatase on Ap4A (left panel) or Ap5A (right panel) obtained from ATP or p4A, catalyzed by acyl-CoA synthetase. Reaction mixtures containing 50 mM Tris-HCl (pH 7.5), 1 mM MgCl2, and purified (see Materials and Methods) Ap4A or Ap5A (33 μM) were treated with asymmetrical dinucleoside tetraphosphatase (0.4 or 0.7 mU/ml, respectively). At the times indicated, aliquots were taken and analyzed by HPLC.
The optimum pH value found for the synthesis of Ap4A from ATP was 5.5, and the Km value for ATP (determined at pH 5.5 and with 1 mM free Mg2+) was 1.2 mM (results not shown).
(vii) Synthesis of heterodinucleoside polyphosphates from ATP and NTP.
In the synthesis of Ap4A, there are formation of an intermediate complex (E · acyl-AMP and/or E-AMP) and transfer of its adenylyl moiety to ATP yielding Ap4A, with Km values for ATP as adenylyl donor and acceptor of 0.015 and 1.2 mM, respectively. As in the case of luciferase, we supposed that the second step for the synthesis of Ap4A by acyl-CoA synthetase could also be rather unspecific, i.e., any NTP could be acceptor of the adenylyl moiety yielding the corresponding Ap4N compound. To diminish the transfer of AMP to another ATP and favor the synthesis of Ap4Ns, we tested the synthesis of Ap4G and Ap4C with a relatively low concentration of ATP (0.63 mM) and relatively high concentrations (3 mM) of GTP and CTP. In these conditions, synthesis of Ap4G and Ap4C and almost no synthesis of Ap4A were observed (data not shown). The identity of the corresponding Ap4N was assessed by its chromatographic behavior in HPLC and UV spectra (Fig. 5). In the case of Ap4G, the identity was also assessed by insensitivity to alkaline phosphatase and by phosphodiesterase treatment (data not shown).
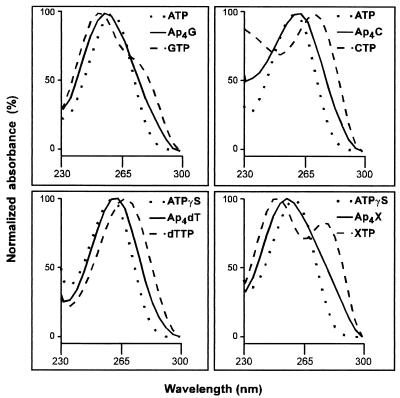
FIG. 5.
Spectra of dinucleoside polyphosphates synthesized by acyl-CoA synthetase. Ap4G and Ap4C were synthesized as described in Materials and Methods; Ap4dT and Ap4X were synthesized as described for Fig. 6. These spectra were obtained with HPLC ChemStation (Hewlett-Packard) from the files produced by the same program during the analysis of the reaction mixtures by HPLC.
(viii) Synthesis of dinucleoside polyphosphates with ATPγS or octanoyl-AMP.
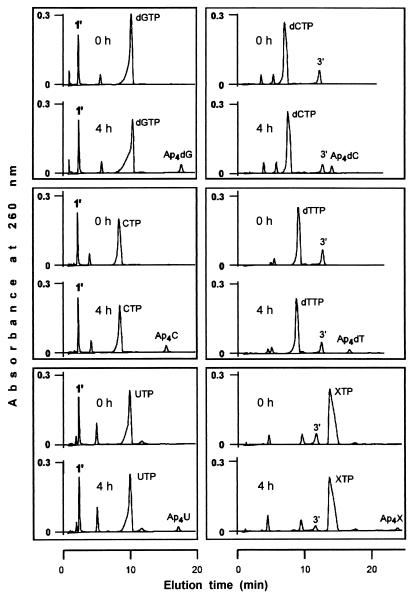
The experiments described below were performed based on our previous experience with luciferase (27). Incubation of acyl-CoA synthetase with only ATPγS or only octanoyl-AMP as substrate failed to produce any dinucleoside polyphosphate; however, when in addition to either of these two substrates, the reaction mixtures were supplemented with GTP, dGTP, CTP, dCTP, UTP, XTP, or dTTP, enzyme-dependent synthesis of the corresponding heterodinucleotides (Ap4N) was observed. ATPγS and octanoyl-AMP were thus donors of the adenylyl moiety to the intermediate complex, but not adenylyl acceptors, whereas the opposite occurred with the NTPs used. ADP and AMP were not acceptors, as no Ap3A or Ap2A was produced. In Fig. 6, chromatograms relative to the synthesis of Ap4dG, Ap4C, Ap4U, Ap4dC, Ap4dT, and Ap4X, from octanoyl-AMP or ATPγS and the corresponding NTPs, are shown. The rates of synthesis of these heteronucleotides were of the same order of magnitude reported above for the synthesis of Ap4A and Ap5A. UV spectra for Ap4dT and Ap4X are depicted in Fig. 5.
FIG. 6.
Synthesis of Ap4N with octanoyl-AMP (left panels) or ATPγS (right panels) as adenylyl donor. The reaction mixtures (90 μl) contained 50 mM MES-KOH (pH 5.5), 0.1 mM dithiothreitol, 6 mM MgCl2, 1 mM octanoyl-AMP (peak 1′) or ATPγS (peak 3′), 5 mM NTP, and dialyzed acyl-CoA synthetase (26 μg of protein). At the times indicated, aliquots were withdrawn and analyzed by HPLC.
(ix) Effect of CoA and fatty acids on the synthesis of p4A.
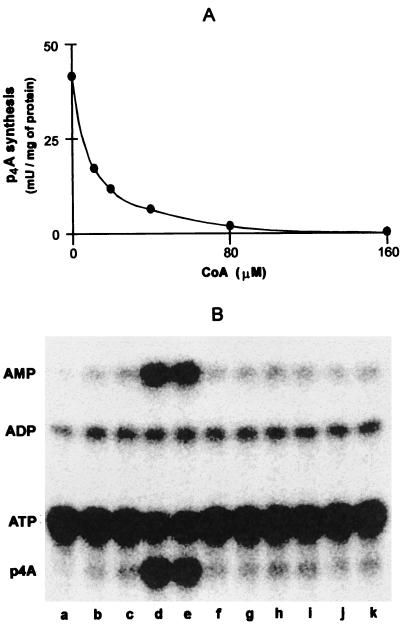
In preliminary experiments, no effect of fatty acids on the synthesis of p4A was observed. We therefore tested whether CoA had an effect. Figure 7A shows that addition of 80 μM CoA nearly abolished activity. The inhibition was reversed by addition of palmitic or octanoic acids, but not by the addition of several other compounds, including acetic acid, several amino acids (lysine, methionine, phenylalanine, and tryptophan), or luciferin at threefold-higher concentrations (Fig. 7B). As the amounts of ATP and fatty acids were in excess over CoA, and pyrophosphatase was present, the AMP was formed in stoichiometric amounts with the CoA present. The control assay mixtures were negative for the synthesis of either p4A or AMP (Fig. 7B).
FIG. 7.
Effect of CoA and organic acids on the synthesis of p4A catalyzed by acyl-CoA synthetase. (A) Effect of CoA on the synthesis of p4A. The reaction mixture (50 μl) contained 50 mM MES-KOH (pH 6.3), 0.1 mM dithiothreitol, 11 mM MgCl2, 1 mM [2,8-3H]ATP, 10 mM P3, and the indicated concentrations of CoA and acyl-CoA synthetase (8.3 μg of protein). (B) Effect of organic acids on the inhibitory effect of CoA on the synthesis of p4A. Reaction mixtures (28 μl) containing 72 mM MES-KOH (pH 6.3), 0.14 mM dithiothreitol, 7.9 mM MgCl2, 0.76 mM [α-32P]ATP, 7.2 mM P3, 0.14 mM CoA, 0.7 μl of desalted inorganic pyrophosphatase, and acyl-CoA synthetase (5.2 μg of protein) were preincubated at 30°C for 20 min. Thereafter, they were supplemented with 12 μl of the following solutions: water (lane b), 1% Triton X-100–5% ethanol (solution C; lane c), 1 mM solutions of palmitic acid in solution C (lane d), octanoic acid in water (lane e), or other possible effectors (acetic acid, lysine, methionine, phenylalanine, tryptophan, and luciferin; lanes f to k, respectively). One hour after the addition of organic acids, aliquots of the reaction were analyzed by TLC. Control without acyl-CoA synthetase is shown in lane a.
DISCUSSION
Acyl-CoA synthetase is in many aspects similar to luciferase and seems to fit into the hypothesis put forward in 1990 (17) that enzymes that catalyze the transfer of a nucleotidyl moiety, forming nucleotidyl-containing intermediates and releasing PPi, may produce dinucleoside polyphosphates. Both enzymes catalyze the synthesis of an intermediate complex (E · LH2-AMP, E-AMP, or E · acyl-AMP [see below]); ATP, dATP, and p4A, nucleotides with a nonmodified α-phosphate (27, 40), are almost equally good substrates for the formation of these intermediates. In a second step, the adenylyl moiety of the complex is transferred to P3 or ATP, yielding p4A or Ap4A, respectively. This second step is rather unspecific, and any NTP (with an intact terminal PPi) can accept the adenylyl moiety of the respective enzyme intermediate complex (40). Both enzymes prefer P3 to ATP as an acceptor substrate (27). In another respect, both enzymes catalyze the synthesis of acyl-CoA compounds, i.e., fatty acyl-CoA for acyl-CoA synthetase (8, 20) and dehydroluciferyl-CoA for luciferase (2, 11). The similarity in the reactions catalyzed by both enzymes is related to the similarity in the amino acid sequences (1). It should be mentioned that functional similarities among acyl-CoA synthetases, aminoacyl-tRNA synthetases, and luciferase were already envisaged by McElroy et al. in 1967 (25).
Formation of enzyme-bound acetyl-AMP by acetyl-CoA synthetase was demonstrated by Berg (7), who proposed the following mechanism (A) for the synthesis of acetyl-CoA:
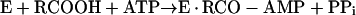 |
reaction 5 |
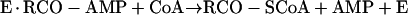 |
reaction 6 |
This sequence of reactions seems to operate in the activation of both short-chain and medium-chain fatty acids (8, 24, 42). However, the mechanism for long-chain fatty acid activation is not yet clear (4–6, 28, 29, 33) and may follow a mechanism (B) where the split of ATP into AMP and PPi does not depend on the presence of a fatty acid (28, 29):
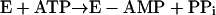 |
reaction 7 |
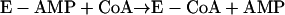 |
reaction 8 |
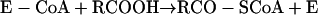 |
reaction 9 |
Accordingly, the synthesis of p4A (or Ap4A) may or may not depend on the presence of an essential effector for the reaction (a fatty acid) resulting from reaction 10 or 11, respectively:
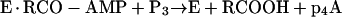 |
reaction 10 |
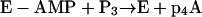 |
reaction 11 |
The commercial acyl-CoA synthetase preparations from P. fragi catalyze the synthesis of p4A without addition of fatty acids to the reaction mixture. This synthesis could take place through mechanism A (reaction 10), by using traces of fatty acids present as contaminants in the reaction mixture, or through mechanism B (reaction 11), in which case the presence of fatty acids is not needed. Accordingly, the inhibitory effect of CoA on the synthesis of p4A (Fig. 7A) and the activator effect of fatty acids (Fig. 7B) added to an assay mixture containing adequate concentrations of CoA could be interpreted in two ways: (i) CoA removes the contaminating fatty acids, essential effectors for the synthesis of p4A from the assay mixture (reactions 5 and 6), or (ii) CoA sequesters the enzyme in the form of E-CoA (reactions 7 and 8), and the addition of fatty acids, liberating free enzyme (reaction 9), allows the synthesis of p4A to take place (reaction 11).
ACKNOWLEDGMENTS
We thank Olga González, Isabel de Diego, and Jara Llenas for very able technical assistance.
This work was supported by a grant from the Dirección General de Investigación Científica y Técnica (PM95/13). R.F. was supported by a Fellowship from Junta Nacional de Investigação Científica and Tecnológica (Portugal).
Footnotes
Dedicated to José Pinto de Barros, retired professor of Physiological Chemistry in the Faculdade de Medicina do Porto, Porto, Portugal.
REFERENCES
- 1.Abe T, Fujino T, Fukuyama R, Minoshima S, Shimizu N, Toh H, Suzuki H, Yamamoto T. Human long-chain acyl-CoA synthetase: structure and chromosomal location. J Biochem. 1992;111:123–128. doi: 10.1093/oxfordjournals.jbchem.a123707. [DOI] [PubMed] [Google Scholar]
- 2.Airth R L, Rhodes W C, McElroy W D. The function of coenzyme A in luminescence. Biochim Biophys Acta. 1958;27:519–532. doi: 10.1016/0006-3002(58)90381-0. [DOI] [PubMed] [Google Scholar]
- 3.Barnes L D, Garrison P N, Siprashvili Z, Guranowski A, Robinson A K, Ingram S W, Croce C M, Ohta M, Huebner K. Fhit, a putative tumor suppressor in humans, is a dinucleoside 5′, 5′′′-P1,P3-triphosphate hydrolase. Biochemistry. 1996;35:11529–11535. doi: 10.1021/bi961415t. [DOI] [PubMed] [Google Scholar]
- 4.Bar-Tana J, Rose G, Shapiro B. Microsomal palmitoyl coenzyme A synthetase from rat liver. Partial and exchange reactions. Biochem J. 1972;129:1101–1107. doi: 10.1042/bj1291101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bar-Tana J, Rose G, Shapiro B. Palmitoyl-coenzyme A synthetase. Isolation of an enzyme-bound intermediate. Biochem J. 1973;135:411–416. doi: 10.1042/bj1350411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bar-Tana J, Rose G, Brandes R, Shapiro B. Palmitoyl-coenzyme A synthetase. Mechanism of reaction. Biochem J. 1973;131:199–209. doi: 10.1042/bj1310199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berg P. Acyl adenylates: an enzymatic mechanism of acetate activation. J Biol Chem. 1956;222:991–1013. [PubMed] [Google Scholar]
- 8.Bremer J, Osmundsen H. Fatty acid oxidation and its regulation. In: Numa S, editor. Fatty acid metabolism and its regulation. Amsterdam, The Netherlands: Elsevier Science Publishers; 1984. pp. 113–154. [Google Scholar]
- 9.Cameselle J C, Costas M J, Günther Sillero M A, Sillero A. Two low Km hydrolytic activities on dinucleoside 5′,5′′′-P1,P4-tetraphosphates in rat liver. Characterization as the specific dinucleoside tetraphosphatase and a phosphodiesterase I-like enzyme. J Biol Chem. 1984;259:2879–2885. [PubMed] [Google Scholar]
- 10.Flodgaard H, Klenow H. Abundant amounts of diadenosine 5′,5′′′-P1,P4-tetraphosphate are present and releasable, but metabolically inactive, in human platelets. Biochem J. 1982;208:737–742. doi: 10.1042/bj2080737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fontes R, Dukhovich A, Sillero A, Günther Sillero M A. Synthesis of dehydroluciferin by firefly luciferase. Effect of dehydroluciferin, coenzyme A and nucleoside triphosphates on the luminescent reaction. Biochem Biophys Res Commun. 1997;237:445–450. doi: 10.1006/bbrc.1997.7161. [DOI] [PubMed] [Google Scholar]
- 12.Frenkel E P, Kitchens R L. Purification and properties of acetyl coenzyme A synthetase from baker’s yeast. J Biol Chem. 1977;252:504–507. [PubMed] [Google Scholar]
- 13.Garrison P N, Barnes L D. Determination of dinucleoside polyphosphates. In: McLennan A G, editor. Ap4A and other dinucleoside polyphosphates. Boca Raton, Fla: CRC Press, Inc.; 1992. pp. 29–61. [Google Scholar]
- 14.Grummt F. Diadenosine 5′,5′′′-P1,P4-tetraphosphate triggers initiation of in vitro DNA replication in baby hamster kidney cells. Proc Natl Acad Sci USA. 1978;75:371–375. doi: 10.1073/pnas.75.1.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guranowski A, Sillero A. Enzymes cleaving dinucleoside polyphosphates. In: McLennan A G, editor. Ap4A and other dinucleoside polyphosphates. Boca Raton, Fla: CRC Press, Inc.; 1992. pp. 81–133. [Google Scholar]
- 16.Guranowski A, Günther Sillero M A, Sillero A. Adenosine 5′-tetraphosphate and adenosine 5′-pentaphosphate are synthesized by yeast acetyl coenzyme A synthetase. J Bacteriol. 1994;176:2986–2990. doi: 10.1128/jb.176.10.2986-2990.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guranowski A, Sillero M A G, Sillero A. Firefly luciferase synthesizes P1,P4-bis(5′-adenosyl)tetraphosphate (Ap4A) and oth- er dinucleoside polyphosphates. FEBS Lett. 1990;271:215–218. doi: 10.1016/0014-5793(90)80409-c. [DOI] [PubMed] [Google Scholar]
- 18.Ignarro L J, Gross R A, Gross D M. Inhibition of mammalian soluble guanylate cyclase activity by adenosine 5′-tetraphosphate, guanosine 5′-tetraphosphate and other nucleotides. J Cyclic Nucleotide Res. 1976;2:337–346. [PubMed] [Google Scholar]
- 19.Jakubowski H. Sporulation of the yeast Saccharomyces cerevisiae is accompanied by synthesis of adenosine 5′-tetraphosphate and adenosine 5′-pentaphosphate. Proc Natl Acad Sci USA. 1986;83:2378–2382. doi: 10.1073/pnas.83.8.2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kornberg A, Pricer W E. Enzymatic synthesis of the coenzyme A derivatives of long chain fatty acids. J Biol Chem. 1953;204:329–343. [PubMed] [Google Scholar]
- 21.Lee J W, Kong I D, Park K S, Jeong S W. Effects of adenosine tetraphosphate (ATPP) on vascular tone in the isolated rat aorta. Yonsei Med J. 1995;36:487–496. doi: 10.3349/ymj.1995.36.6.487. [DOI] [PubMed] [Google Scholar]
- 22.Lobatón C D, Vallejo C G, Sillero A, Sillero M A G. Diguanosinetetraphosphatase from rat liver: activity on diadenosine tetraphosphate and inhibition by adenosine tetraphosphate. Eur J Biochem. 1975;50:495–501. doi: 10.1111/j.1432-1033.1975.tb09888.x. [DOI] [PubMed] [Google Scholar]
- 23.Lundin A, Rickardsson A, Thore A. Continuous monitoring of ATP-converting reactions by purified firefly luciferase. Anal Biochem. 1976;75:611–620. doi: 10.1016/0003-2697(76)90116-0. [DOI] [PubMed] [Google Scholar]
- 24.Mao L, Millington D S, Schulz H. Formation of free acyl adenylate during activation of 2-propylpentanoic acid. Valproyl-AMP: a novel cellular metabolite of valproic acid. J Biol Chem. 1992;267:3143–3146. [PubMed] [Google Scholar]
- 25.McElroy W D, DeLuca M, Travis J. Molecular uniformity in biological catalyses. The enzymes concerned with firefly luciferin, amino acid, and fatty acid utilization are compared. Science. 1967;157:150–160. doi: 10.1126/science.157.3785.150. [DOI] [PubMed] [Google Scholar]
- 26.Ogilvie A. Extracellular functions for ApnA. In: McLennan A G, editor. Ap4A and other dinucleoside polyphosphates. Boca Raton, Fla: CRC Press, Inc.; 1992. pp. 229–273. [Google Scholar]
- 27.Ortiz B, Sillero A, Günther Sillero M A. Specific synthesis of adenosine(5′)tetraphospho(5′)nucleoside and adenosine(5′)oligophospho(5′)adenosine (n > 4) catalyzed by firefly luciferase. Eur J Biochem. 1993;212:263–270. doi: 10.1111/j.1432-1033.1993.tb17658.x. [DOI] [PubMed] [Google Scholar]
- 28.Parsons P, Huang S. Mechanism of action of long chain fatty acyl-CoA ligase from rat liver mitochondria. J Cell Biol. 1977;75:307a. [Google Scholar]
- 29.Philipp D P, Parsons P. Kinetic characterization of long chain fatty acyl coenzyme A ligase from rat liver mitochondria. J Biol Chem. 1979;254:10785–10790. [PubMed] [Google Scholar]
- 30.Plateau P, Blanquet S. Synthesis of NpnN (n = 3 or 4) in vitro and in vivo. In: McLennan A G, editor. Ap4A and other dinucleoside polyphosphates. Boca Raton, Fla: CRC Press, Inc.; 1992. pp. 63–79. [Google Scholar]
- 31.Remy P. Intracellular functions of ApnN: eukaryotes. In: McLennan A G, editor. Ap4A and other dinucleoside polyphosphates. Boca Raton, Fla: CRC Press, Inc.; 1992. pp. 151–204. [Google Scholar]
- 32.Rodríguez del Castillo A, Torres M, Delicado E G, Miras-Portugal M T. Subcellular distribution studies of diadenosine polyphosphates -Ap4A and Ap5A- in bovine adrenal medulla: presence in chromaffin granules. J Neurochem. 1988;51:1696–1703. doi: 10.1111/j.1471-4159.1988.tb01147.x. [DOI] [PubMed] [Google Scholar]
- 33.Rose G, Bar-Tana J, Shapiro B. Palmitoyl coenzyme A synthetase activation by uncomplexed ATP. Biochim Biophys Acta. 1979;573:126–135. doi: 10.1016/0005-2760(79)90179-6. [DOI] [PubMed] [Google Scholar]
- 34.Ruiz A, Hurtado C, Ribeiro J M, Sillero A, Günther Sillero M A. Hydrolysis of bis(5′-nucleosidyl)polyphosphates by Escherichia coli 5′-nucleotidase. J Bacteriol. 1989;171:6703–6709. doi: 10.1128/jb.171.12.6703-6709.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schlüter H, Offers E, Brüggemann G, van-der-Giet M, Tepel M, Nordhoff E, Karas M, Spieker C, Witzel H, Zidek W. Diadenosine phosphates and the physiological control of blood pressure. Nature. 1994;367:186–188. doi: 10.1038/367186a0. [DOI] [PubMed] [Google Scholar]
- 36.Sillero A, Günther Sillero M A. Purine nucleotide metabolism in Artemia. In: MacRae T H, Bagshaw J C, Warner A H, editors. Biochemistry and cell biology of Artemia. Boca Raton, Fla: CRC Press, Inc.; 1989. pp. 95–111. [Google Scholar]
- 37.Sillero M A G, Cameselle J C. Interactions of dinucleoside polyphosphates with enzymes and other proteins. In: McLennan A G, editor. Ap4A and other dinucleoside polyphosphates. Boca Raton, Fla: CRC Press, Inc.; 1992. pp. 205–228. [Google Scholar]
- 38.Sillero M A G, Del-Valle M, Zaera E, Michelena P, García A G, Sillero A. Diadenosine 5′,5′′-P1,P4-tetraphosphate (Ap4A), ATP and catecholamine content in bovine adrenal medulla, chromaffin granules and chromaffin cells. Biochimie. 1994;76:404–409. doi: 10.1016/0300-9084(94)90116-3. [DOI] [PubMed] [Google Scholar]
- 39.Sillero M A G, Madrid O, Zaera E, Sillero A. 2′,3′-Dideoxynucleoside triphosphates (ddNTP) and di-2′,3′-dideoxynucleoside tetraphosphates (ddNp4ddN) behave differently to the corresponding NTP and Np4N counterparts as substrates of firefly luciferase, dinucleoside tetraphosphatase and phosphodiesterases. Biochim Biophys Acta. 1997;1334:191–199. doi: 10.1016/s0304-4165(96)00092-x. [DOI] [PubMed] [Google Scholar]
- 40.Sillero M A G, Guranowski A, Sillero A. Synthesis of dinucleoside polyphosphates catalyzed by firefly luciferase. Eur J Biochem. 1991;202:507–513. doi: 10.1111/j.1432-1033.1991.tb16402.x. [DOI] [PubMed] [Google Scholar]
- 41.Small G, Cooper C. Studies on the occurrence and biosynthesis of adenosine tetraphosphate. Biochemistry. 1966;5:26–33. doi: 10.1021/bi00865a004. [DOI] [PubMed] [Google Scholar]
- 42.Webster L T, Campagnari F. The biosynthesis of acetyl and butyryl adenylates. J Biol Chem. 1962;237:1050–1055. [PubMed] [Google Scholar]
- 43.Whitehouse M, Moeksi H, Gurin S. The synthesis and biological properties of fatty acyl adenylates. J Biol Chem. 1957;226:813–819. [PubMed] [Google Scholar]
- 44.Zamecnik P C, Stephenson M L. Nucleoside pyrophosphate compounds related to the first step in protein synthesis. Alfred Benzon Found Symp. 1969;I:276–291. [Google Scholar]
- 45.Zamecnik P C, Stephenson M L, Janeway C M, Randerath K. Enzymatic synthesis of diadenosine tetraphosphate and diadenosine triphosphate with a purified lysyl-sRNA synthetase. Biochem Biophys Res Commun. 1966;24:91–97. doi: 10.1016/0006-291x(66)90415-3. [DOI] [PubMed] [Google Scholar]