Figure 8.
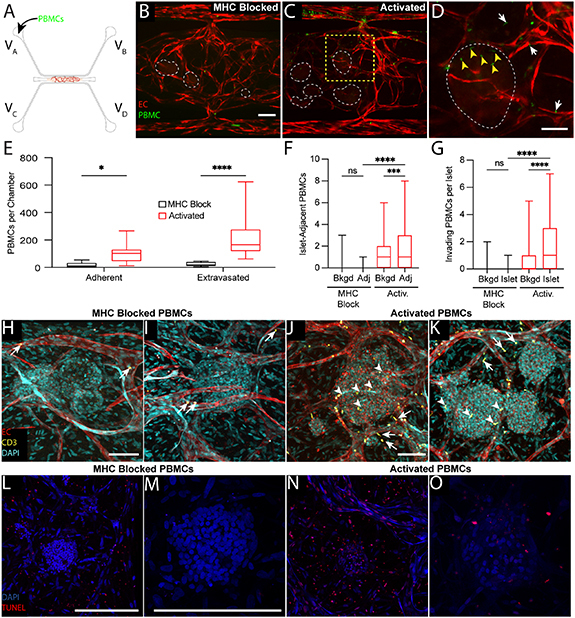
Immune cell and islet interactions are modeled within the islet-VMO. (A) CellTracker+ MHC-blocked or activated PBMCs were added to reservoir VA and allowed to perfuse though islet-VMOs containing islets from the same donor for up to 48 h. (B) MHC-blocked PBMCs (green) demonstrate minimal adhesion or extravasation from vessels (fluorophore-transduced ECs, red) as compared to (C) activated PBMCs (islets, dashed outline). Scale bar = 200 μm. (D) Inset from (C) (yellow dashed box) shows increased migration of activated PBMCs out of the vasculature towards the islet (white arrows) with multiple PBMCs co-localizing within the islet itself (yellow arrowheads). (E) Quantification of PBMC staining shows increased adhesion and extravasation of activated but not MHC-blocked PBMCS across multiple islet donors (two-way ANOVA, *p < 0.05, ****p < 0.0001) (n = 14 Islet-VMOs across 4 islet donors). (F) The number of PBMCs within 100 μm of an islet was quantified (Adj) and compared to both background, non-islet regions (Bkgd) and MHC-blocked PBMCs (one-way ANOVA, ***p < 0.001, ****p < 0.0001) (n > 65 islets across 4 islet donors). (G) The number of PBMCs present within islets (islet) was quantified and compared to PBMC counts in background, non-islet regions (Bkgd) and MHC-blocked PBMCs. (one-way ANOVA, ***p < 0.001, ****p < 0.0001 by) (n > 65 islets across 4 islet donors). (H), (I) Confocal imaging of fixed and stained islet-VMOs shows minimal adhesion (arrows) of perfused with MHC-blocked CD3+ T cells (green) to the vessels (CD31+, red; DAPI, cyan). (J), (K) Immunofluorescent staining of islet-VMOs perfused with activated PBMCs shows increased extravasation (white arrows) and islet (DAPI, cyan) invasion (yellow arrowheads) of CD3+ T cells. (L,M) TUNEL staining of islet-VMOs perfused with MHC-blocked-PBMCs shows little to no cell death in the islets. Scale bars, 100 μm. (N), (O) TUNEL staining of islet-VMOs perfused with activated PBMCs shows evidence of cell death in the islet. Scale bars, 100 μm.
