Recently, the SELECT (Semaglutide Effects on Cardiovascular Outcomes in People with Overweight or Obesity) trial released its results, indicating that once-weekly semaglutide 2.4 mg reduces the risk of major cardiovascular events by 20% in adults aged ≥45 years with obesity, established cardiovascular diseases (CVDs), and no history of diabetes.1–3 While semaglutide is currently approved by the Food and Drug Administration for chronic weight management in individuals with overweight or obesity and at least 1 weight-related condition, it has not been approved specifically for reducing cardiovascular risk.4 The SELECT trial highlights its potential for secondary CVD prevention. In this study, we investigate the eligibility of the US population for semaglutide-based secondary CVD prevention based on the SELECT trial, aiming to provide a general estimate of those who might benefit from this treatment. Additionally, we examine race, ethnicity, and socioeconomic status (SES) to gain insights into how improving access to these medications for marginalized populations or those with low SES could help reduce disparities.
The data that support the findings of this study are available from the corresponding author upon reasonable request. We conducted a cross-sectional analysis using data from the National Health and Nutrition Examination Survey, encompassing 4 cycles from 2011 to 2012 to from 2017 to 2020. Eligibility was defined as consistent with the main SELECT trial criteria. Instead of exactly replicating the SELECT criteria, which includes various uncommon exclusions (eg, chronic pancreatitis), we focused on individuals aged ≥45 years with a body mass index ≥27 kg/m2 and established atherosclerotic CVD (self-reported physician diagnoses of coronary heart disease, heart attack, or stroke). We did not include individuals with symptomatic peripheral artery disease because this information is not available in the National Health and Nutrition Examination Survey from 2011 to 2020. We excluded individuals with diabetes based on a glycated hemoglobin level of 6.5% or higher or treatment with any anti-diabetes medication.
Across each cycle, we calculated age-sex–adjusted percentages of eligible participants for semaglutide treatment consistent with the main SELECT criteria,1–3 with further stratification by race, ethnicity, and socioeconomic indicators, including family income, educational attainment, health insurance, and employment status and marital status. We used methods appropriate for structured survey data to produce nationally representative estimates.5 Participants with missing covariate data were excluded from the respective subgroup analysis. All analyses were conducted using R 4.3, and this study received an exemption from the Yale University Institutional Review Board.
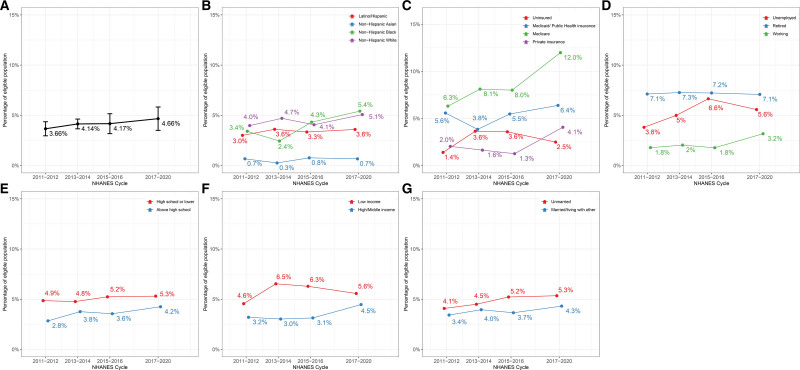
Among the 15 399 adults, the mean age was 60.9 (SD, 10.5) years. Of these, 53.1% (52.1–54.0) identified as women, 11.0% (9.1–12.9) as Hispanic/Latino, 5.0% (4.0–5.9) as non-Hispanic Asian, 10.6% (8.8–12.4) as non-Hispanic Black, and 73.4% (70.3–76.6) as non-Hispanic White adults. The population estimates of individuals meeting the main SELECT criteria increased from 4.4 million (95% CI, 3.6-5.3) from 2011 to 2012 to 6.2 million (95% CI, 4.7-7.8) from 2017 to 2020. There was a concurrent increase in the proportion of eligible individuals (Figure [A]), and by 2017 to 2020, 4.7% (95% CI, 3.5-5.8) of US adults met the main SELECT inclusion criteria.
Figure.
Age-sex–adjusted percentage of individuals aged ≥45 years eligible for semaglutide and overall population and by racial and ethnic and socioeconomic subgroups. A, Eligibility for overall population. B, Eligibility by race and ethnicity. C, Eligibility by health insurance. D, Eligibility by employment status. E, Eligibility by education level. F, Eligibility by family income level. G, Eligibility by marital status. The body mass index cutoff for Asian individuals may be lower than other racial and ethnic subgroups. However, it is worth noting that clinical trials and regulatory agencies have not universally considered the lower cutoff for Asian individuals, and there is a lack of consensus on the specific body mass index thresholds that should or could be used. NHANES indicates National Health and Nutrition Examination Survey.
When stratified by race and ethnicity, significant differences in eligibility percentages emerged (Figure [B]). From 2011 to 2020, White adults had the highest eligibility rate at 4.65% (4.04–5.27; 3.68 million), followed closely by Black adults at 4.06% (3.45–4.67; 0.46 million), Hispanic/Latino adults at 3.42% (2.81–4.03; 0.40 million), and Asian adults at 0.67% (0.31–0.10;0.04 million).
Significant differences were also evident when considering SES indicators (Figure [C through G]). Adults with lower educational attainment exhibited a higher eligibility percentage at 5.18% (4.41-5.96; 2.20 million) compared with more-educated counterparts at 3.82% (3.29-4.35; 2.60 million). Additionally, eligibility was higher among those unemployed or not in the labor force, standing at 5.36% (4.32, 6.40; 1.11 million), than among those employed, where it was 2.34% (1.74, 2.95; 1.34 million). Individuals from lower income families were also more likely to meet eligibility criteria, with a rate of 5.83% (5.13-6.53, 1.85 million), compared with those from middle/high-income families that had a rate of 3.72% (3.13-4.30, 2.58 million). Importantly, across all sociodemographic subgroups, the proportion of eligible individuals increased over time.
Millions of Americans would be eligible and could potentially benefit from semaglutide-based secondary CVD prevention. This underscores the potential of semaglutide in reducing cardiovascular risk for those with obesity and CVD without diabetes. However, the promise of this breakthrough is clouded by the reality that many eligible individuals may find this therapy inaccessible due to access and financial barriers. If made available and affordable, these medications could help address disparities, particularly among marginalized groups and those with lower SES.
Limitations include declining National Health and Nutrition Examination Survey response rates and potential overestimation due to missing data on uncommon exclusions, such as chronic pancreatitis and peripheral artery disease during the study period.
In conclusion, the SELECT trial represents a pivotal advancement in secondary cardiovascular prevention for those with overweight or obesity and CVD without diabetes. The increasing number of this group over the past decade, coupled with overrepresentation among minoritized populations and lower SES individuals, highlights the urgent need to address access challenges.
ARTICLE INFORMATION
Sources of Funding
None.
Disclosures
Drs Lu and Krumholz had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Dr Jastreboff conducts multicenter trials with Eli Lilly, Novo Nordisk, and Rhythm Pharmaceuticals; serves on scientific advisory boards for Amgen, AstraZeneca, Boehringer Ingelheim, Biohaven, Eli Lilly, Intellihealth, Novo Nordisk, Pfizer, Rhythm Pharmaceuticals, Scholar Rock, Structure Therapeutics, Terms Pharmaceutical, WeightWatchers, and Zealand Pharmaceuticals; was the site principal investigator for the SELECT (Semaglutide Effects on Cardiovascular Outcomes in People with Overweight or Obesity) trial at Yale University; and receives institutional grant funding from the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health. Dr Rodriguez reports consulting relationships with Healthpals, Novartis, Esperion, NovoNordisk (Clinical Events Committee), Movano Health, and Kento Health. In the past three years, Harlan Krumholz received expenses or personal fees from Element Science, Eyedentify, and F-Prime. He is a co-founder of Hugo Health, Refactor Health, and Ensight-AI. He is the co-editor of Journal Watch: Cardiology of the Massachusetts Medical Society and is a section editor of UpToDate. He is associated with contracts, through Yale New Haven Hospital, from the Centers for Medicare & Medicaid Services and through Yale University from Janssen, Johnson & Johnson Consumer, and Pfizer. Dr. Lu received support from the Sentara Research Foundation, the National Heart, Lung, and Blood Institute of the National Institutes of Health (under awards R01HL69954 and R01HL169171), and the Patient-Centered Outcomes Research Institute (under award HM-2022C2-28354). Dr. Khera is an Associate Editor of JAMA. He receives support from the National Heart, Lung, and Blood Institute of the National Institutes of Health (under award K23HL153775) and the Doris Duke Charitable Foundation (under award 2022060). He also receives research support, through Yale, from Bristol-Myers Squibb and Novo Nordisk. He is a coinventor of U.S. Provisional Patent Applications 63/177,117, 63/428,569, 63/346,610, 63/484,426, and 63/508,315. He is a co-founder of Ensight-AI and Evidence2Health, health platforms to improve cardiovascular diagnosis and evidence-based cardiovascular care. All data used in this study are publicly available at https://www.cdc.gov/nchs/nhanes/index.htm. The other authors report no conflicts.
Nonstandard Abbreviations and Acronyms
- CVD
- cardiovascular disease
- SES
- socioeconomic status
For Sources of Funding and Disclosures, see page 84.
Contributor Information
Yuan Lu, Email: y.lu@yale.edu.
Yuntian Liu, Email: yuntian.liu@yale.edu.
Ania M. Jastreboff, Email: ania.jastreboff@yale.edu.
Rohan Khera, Email: rohan.khera@yale.edu.
Fatima Rodriguez, Email: frodrigu@stanford.edu.
Karol E. Watson, Email: kwatson@mednet.ucla.edu.
REFERENCES
- 1.Lincoff AM, Brown-Frandsen K, Colhoun HM, et al. Semaglutide and cardiovascular outcomes in obesity without diabetes. N Engl J Med. doi: 10.1056/NEJMoa2307563 [DOI] [PubMed] [Google Scholar]
- 2.Ryan DH, Lingvay I, Colhoun HM, Deanfield J, Emerson SS, Kahn SE, Kushner RF, Marso S, Plutzky J, Brown-Frandsen K, et al. Semaglutide effects on cardiovascular outcomes in people with overweight or obesity (SELECT) rationale and design. Am Heart J. 2020;229:61–69. doi: 10.1016/j.ahj.2020.07.008 [DOI] [PubMed] [Google Scholar]
- 3.Lingvay I, Brown-Frandsen K, Colhoun HM, Deanfield J, Emerson SS, Esbjerg S, Hardt-Lindberg S, Hovingh GK, Kahn SE, Kushner RF, et al. ; SELECT Study Group. Semaglutide for cardiovascular event reduction in people with overweight or obesity: SELECT study baseline characteristics. Obesity (Silver Spring). 2023;31:111–122. doi: 10.1002/oby.23621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.US Food and Drug Administration. FDA approves new drug treatment for chronic weight management, first since 2014. Accessed October 17, 2023. https://www.fda.gov/news-events/press-announcements/fda-approves-new-drug-treatment-chronic-weight-management-first-2014
- 5.Centers for Disease Control and Prevention. US National Health and Nutrition Examination Survey. Accessed April 17, 2023. http://www.cdc.gov/nchs/nhanes.htm