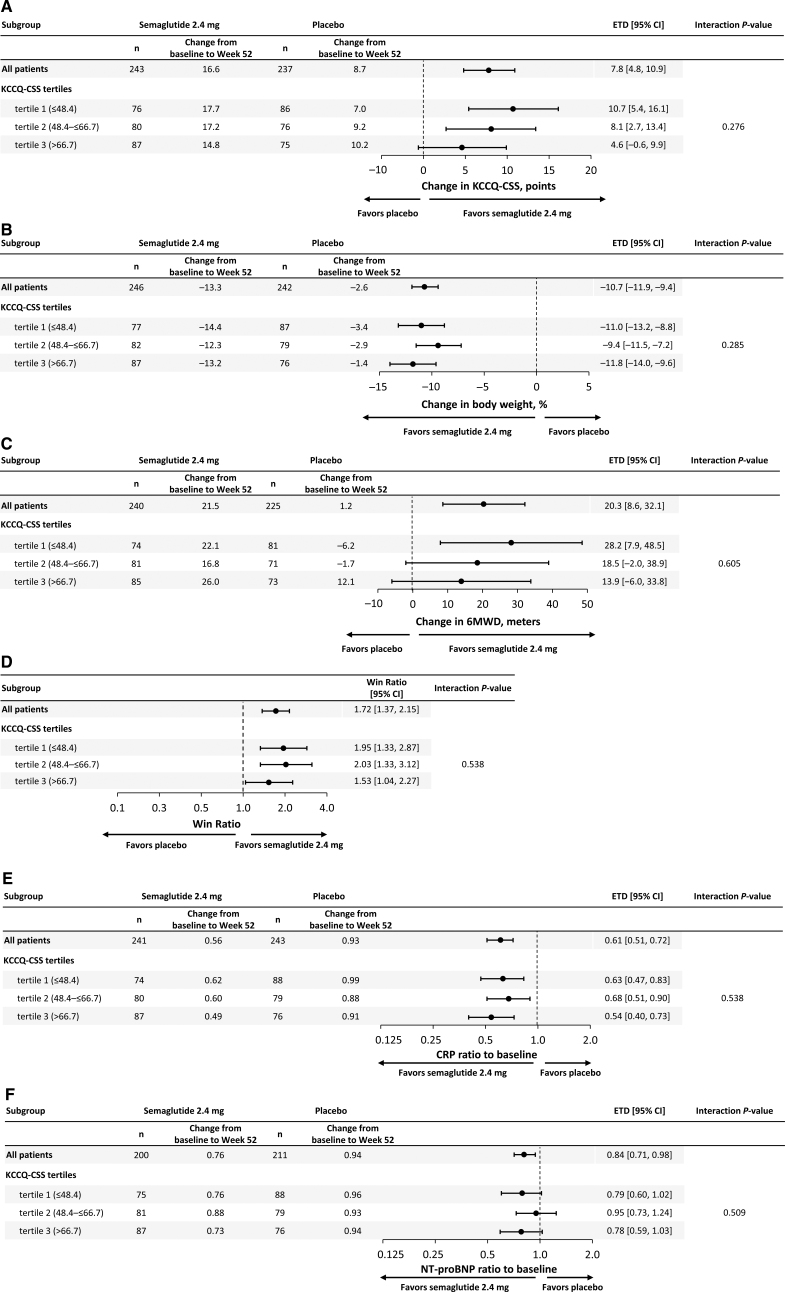
Figure 1.
Effects of semaglutide compared with placebo across KCCQ tertiles on heart failure symptoms and physical limitations (KCCQ-CSS; A); body weight (B); exercise function (6MWD; C); hierarchical composite end point (D); systemic inflammation (CRP; E); and NT-proBNP (F). Data are from the in-trial period for the full analysis set. Week 52 responses were analyzed using an ANCOVA model with randomized treatment, subgroup, and treatment by subgroup interaction as factors and baseline KCCQ-CSS as covariate. Missing observations for reasons other than cardiovascular death or previous heart failure events (if nonretrieved) were multiple (×1000) imputed from retrieved participants of the same randomized treatment arm. Missing observations due to cardiovascular death or previous heart failure events were imputed using a composite strategy with the least favorable value determined during the trial. P values for linear trend across the KCCQ tertiles were: 0.113 (KCCQ-CSS); 0.617 (body weight); 0.330 (6MWD); 0.478 (CRP), and 0.965 (NT-proBNP). 6MWD indicates 6-minute walk distance; CRP, C-reactive protein; CSS, Clinical Summary Score; ETD, estimated treatment difference; KCCQ, Kansas City Cardiomyopathy Questionnaire; and NT-proBNP, N-terminal pro-brain natriuretic peptide.