Fig 5.
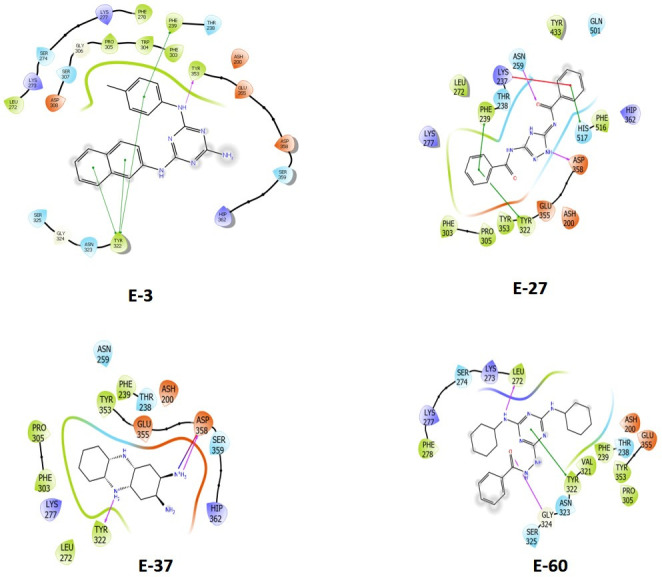
Predicted molecular interactions of the four lead inhibitors with human ENPP1. Molecular docking of inhibitors E-3 (IC50 26.4 µM or 10 µg/mL), E-27 (IC50 16.3 µM or 5 µg/mL), E-37 (IC50 44.6 µM or 10 µg/mL), and E-60 (IC50 9.8 µM or 4 µg/mL) with the active site of ENPP1. Predicted hydrogen bonds are shown with purple arrows, while predicted salt bridges are shown with black arrows. Predicted pi-stacking interactions are shown by green lines, with hydrophobic surfaces of the inhibitors being shaded in gray. Predicted pi-cation interactions are shown with red lines. For the enzyme active site, acidic residues and surfaces are colored orange, basic residues are purple, hydrophobic residues are green, and hydrophilic residues and surfaces are blue.
