ABSTRACT
Early COVID-19 convalescent plasma (CCP) transfusion to outpatients with COVID-19 decreases progression to hospitalization, but the mechanism of how CCP reduces severity is unknown. Among 882 COVID-19 participants transfused with CCP or control plasma in a randomized controlled trial, 21 cytokines and chemokines were measured using electrochemiluminescence assays. Wilcoxon rank sum tests were used to evaluate the difference between early (transfused within 5 days of symptom onset) CCP vs early control plasma and late (transfused 6–9 days after symptom onset) CCP vs late control plasma at each visit. Linear mixed-effect models were used to assess the difference in the slope of cytokine change. Median cytokine and chemokine levels were similar between the early CCP and early control groups pre-transfusion. At the day 14 visit, only the median IL-6 (P = 0.014) and IL-16 (P = 0.036) levels were lower in the early CCP group compared to the early control group, but these differences were not statistically significant after correcting for multiple comparisons (requiring P < 0.0024). IL-6 levels decreased significantly faster in the early CCP group from screening to the day 14 visit compared to the early control group (P < 0.001). No difference was observed in the slope of cytokine change from screening to day 90 between early CCP and early control groups. Late control and late CCP arms showed similar cytokine and chemokine levels through study follow-up. One mechanism by which early CCP transfusion reduces hospitalization may be by decreasing IL-6 levels, as the reduction is associated with better recovery from COVID-19.
IMPORTANCE
This study examined the role that cytokines may have played in the beneficial outcomes found when outpatient individuals infected with SARS-CoV-2 were transfused with COVID-19 convalescent plasma (CCP) early in their infection. We found that the pro-inflammatory cytokine IL-6 decreased significantly faster in patients treated early with CCP. Participants with COVID-19 treated with CCP later in the infection did not have the same effect. This decrease in IL-6 levels after early CCP treatment suggests a possible role of inflammation in COVID-19 progression. The evidence of IL-6 involvement brings insight into the possible mechanisms involved in CCP treatment mitigating SARS-CoV-2 severity.
KEYWORDS: COVID-19, COVID-19 serotherapy, convalescent plasma, SARS-CoV-2, cytokines, chemokines, randomized trial
INTRODUCTION
Inflammation is one of the leading causes of COVID-19 morbidity [e.g., acute respiratory distress syndrome (ARDS)] and mortality (1 – 8). The innate immune activation and cytokine storm are associated with COVID-19 severity (9, 10). Individuals who have recovered from COVID-19 have persistently increased inflammatory cytokines, and elevated levels of IL-6 are associated with post-COVID conditions (11, 12). In addition, it has been shown that vaccination reduces inflammatory markers in recently infected individuals (2).
COVID-19 convalescent plasma (CCP) has been shown to decrease hospitalization if it contains high titers of antibodies against SARS-CoV-2 and is provided early relative to the diagnosis or onset of symptoms (13 – 20). However, the mechanism for CCP to reduce severity is unknown (21). This study compared the levels of 21 different cytokines in participants who received CCP or a control pre-pandemic plasma within 9 days of symptom onset as part of a randomized controlled trial (13).
MATERIALS AND METHODS
Population
The randomized double-blinded placebo-controlled clinical trial population included participants from the Convalescent Plasma to Limit Coronavirus-Associated Complications (CSSC-004) trial (NCT04373460), as previously described (13). The trial excluded participants who were or planned to be hospitalized, had a history of adverse transfusion reactions, received monoclonal antibodies, or were unable to comply with protocols. We transfused 1181 participants with either CCP or SARS-CoV-2 seronegative control plasma up to 9 days after COVID-19 symptom onset from 23 sites in the United States between June 2020 and October 2021. For this study, we included 882 participants with samples and symptom data collected at screening, day 14, and day 90 visits, as previously described (Fig. S1) (2, 11).
The JHU Institutional Review Board (IRB) and the Human Research Protection Office of the United States Department of Defense approved the trial. The Navajo Nation Human Research Review Board and the Indian Health Service National IRB also approved study activities at the Center for Indigenous Health sites. All participants provided written, informed consent.
Data collection
Blood samples were collected in ethylenediaminetetraacetic acid tubes; plasma was separated and stored at −80°C until cytokines were measured (2, 11). Electrochemiluminescence multiplexed sandwich immunoassays were used to quantify the concentration (pg/mL) of 21 different cytokine and chemokine analytes according to the manufacturer’s instructions (MesoScale Discovery, Gaithersburg, MD, USA). Samples and calibrators were run in duplicate (2, 11). Standard curves quantifying cytokine/chemokine concentrations were based on calibrators run with each plate. Both runs of the analytes had to be above the plate-specific lower limit of detection for them to be considered detectable. Cytokine values that were below the lower limit of detection or above the higher limit of detection were extrapolated using standard curves. The duplicates were averaged for analysis. Analytes with a coefficient of variation greater than 25 and a signal greater than 2,000 were considered invalid and rerun (2, 11).
Statistical analysis
The early CCP and early control plasma groups were defined as transfusions occurring at most 5 days after symptom onset. Late CCP and late control plasma groups were defined as transfusions occurring 6 to 9 days after symptom onset.
Missing cytokine values out of the fit curve range were imputed using a stochastic draw from the extrapolated lower or upper tails of the fitted log10 normal distribution of available cytokine values, and outliers were excluded as previously described (2, 11). Cytokine values were log10 transformed, and comparisons were made between early control vs early CCP and late control vs late CCP. Wilcoxon rank sum tests were used to evaluate the difference in cytokine levels between treatment groups at screening (pre-transfusion), day 14 and day 90. Linear mixed-effect models with an unstructured covariance matrix were used to assess the difference in slope of cytokine change between treatment groups from screening to days 14 and 90, adjusting for age, sex, body mass index (BMI), hypertension, diabetes, vaccine status, and COVID-19 waves. We adjusted for multiple comparisons using Bonferroni correction [requiring two-tailed P < 0.0024 (P < 0.05 ÷ 21)]. All analyses were conducted in R 4.2.
RESULTS
Among the 882 trial participants, the majority were female (57.4%), and the median age was 43 years, as previously described (Table S1) (2, 11). There were no significant differences between early and late CCP vs early and late control plasma trial arms, respectively, by age, sex, race, ethnicity, BMI, or vaccine status. Among the participants, 197 (22.3%) participants had CCP and 195 (22.1%) had control plasma transfused within 5 days of symptom onset (i.e., early). For participants transfused more than 5 days since symptom onset (i.e., late), 248 (28.1%) had CCP and 242 (27.4%) had control plasma.
Pre-transfusion, the median cytokine and chemokine levels were similar between the early CCP and the early control group. The median IL-2 receptor alpha chain (IL-2RA) levels were lower among the late CCP than the late control group (P = 0.008), but the difference was not statistically significant after adjusting for multiple comparisons (requiring P < 0.0024) (Fig. S2).
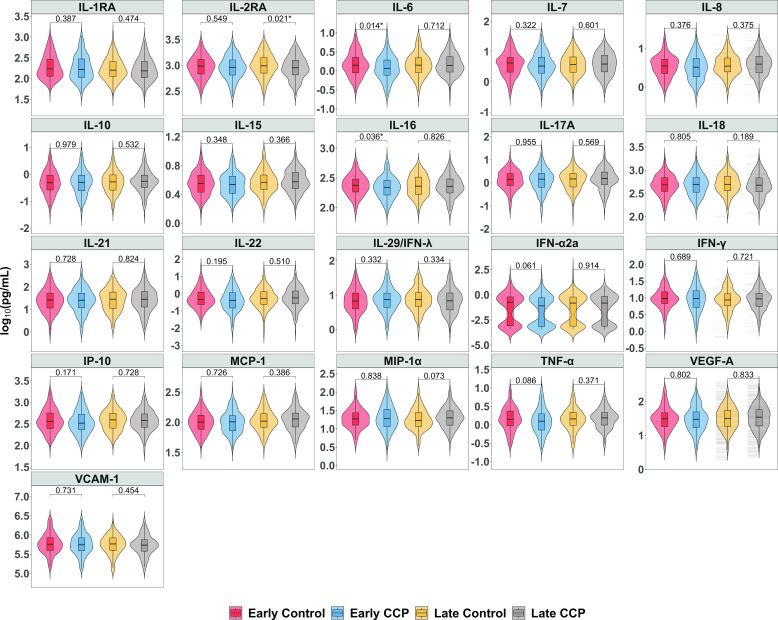
At the day 14 visit, median IL-6 (P = 0.014) and IL-16 (P = 0.036) levels were lower among the early CCP group compared to the early control group (Fig. 1), but these differences were not statistically significant after adjusting for multiple comparisons. At the day 90 visit, median IL-16 levels of early CCP remained marginally lower than the early control group (P = 0.010) (Fig. S3).
Fig 1.
Day 14 cytokine and chemokine levels stratified by treatment group. *P < 0.05.
From screening to day 14, levels of IL-1RA, IL-6, IL-15, IL-18, IFN-α2a, and IP-10 decreased faster among the early CCP than the early control group (P < 0.05), but only IL-6 levels decreased significantly faster after adjusting for multiple comparisons (P < 0.001) (Table 1). No difference was observed in the slope of cytokine change from screening to day 90 between early CCP and early control groups.
TABLE 1.
Comparison of the slope of the daily changes of cytokine levels between early control and early CCP
| Difference in slope of change between early control and early CCP (early CCP minus early control) c | ||||
|---|---|---|---|---|
| Screening to day 14 | Screening to day 90 | |||
| Analyte | β (95% CI) | P value | β (95% CI) | P value |
| IL-1RA | −0.0058 (−0.0095, −0.0020) | 0.003 a | −0.0005 (−0.0011, 0.0001) | 0.126 |
| IL-2RA | −0.0001 (−0.0021, 0.0019) | 0.906 | 0.0001 (−0.0003, 0.0004) | 0.748 |
| IL-6 | −0.0100 (−0.0151, −0.0050) | <0.001 b | −0.0002 (−0.0011, 0.0007) | 0.630 |
| IL-7 | −0.0003 (−0.0051, 0.0044) | 0.895 | −0.0001 (−0.0008, 0.0006) | 0.805 |
| IL-8 | −0.0010 (−0.0052, 0.0031) | 0.631 | −0.0001 (−0.0008, 0.0006) | 0.780 |
| IL-10 | −0.0037 (−0.0102, 0.0029) | 0.274 | −0.0002 (−0.0014, 0.0010) | 0.705 |
| IL-15 | −0.0028 (−0.0050, −0.0007) | 0.011 a | −0.0002 (−0.0006, 0.0001) | 0.165 |
| IL-16 | −0.0014 (−0.0036, 0.0008) | 0.215 | −0.0002 (−0.0006, 0.0001) | 0.231 |
| IL-17A | −0.0001 (−0.0068, 0.0066) | 0.985 | −0.0003 (-0.0015, 0.0008) | 0.563 |
| IL-18 | −0.0030 (−0.0054, −0.0007) | 0.012 a | −0.0003 (−0.0007, 0.0001) | 0.159 |
| IL-21 | −0.0023 (−0.0080, 0.0035) | 0.438 | −0.0001 (−0.0011, 0.0008) | 0.750 |
| IL-22 | −0.0088 (−0.0208, 0.0032) | 0.152 | 0.0004 (−0.0009, 0.0016) | 0.566 |
| IL-29/IFN-λ | −0.0023 (−0.0073, 0.0028) | 0.380 | −0.0006 (−0.0014, 0.0002) | 0.165 |
| IFN-α2a | −0.0222 (−0.0441, −0.0003) | 0.047 a | −0.0009 (−0.0048, 0.0030) | 0.645 |
| IFN-γ | −0.0081 (−0.0163, 0.0002) | 0.055 | −0.0010 (−0.0024, 0.0005) | 0.199 |
| IP-10 | −0.0055 (−0.0109, −0.0001) | 0.048 a | −0.0003 (−0.0014, 0.0008) | 0.582 |
| MCP-1 | −0.0004 (−0.0030, 0.0022) | 0.767 | −0.0004 (−0.0008, 0.0000) | 0.084 |
| MIP-1α | −0.0030 (−0.0073, 0.0012) | 0.160 | −0.0002 (−0.0009, 0.0005) | 0.610 |
| TNF-α | −0.0057 (−0.0115, 0.0000) | 0.052 | −0.0002 (−0.0013, 0.0008) | 0.644 |
| VEGF-A | 0.0014 (−0.0029, 0.0057) | 0.536 | 0.0004 (−0.0003, 0.0010) | 0.257 |
| VCAM-1 | 0.0014 (−0.0020, 0.0049) | 0.418 | 0.0001 (−0.0005, 0.0006) | 0.802 |
P < 0.05.
P < 0.0024 (statistically significant after adjusting for multiple comparisons using Bonferroni correction).
The difference in slope (95% CI) of the daily changes of log10 pg/mL values of each analyte during follow-up is presented. Each analyte had a separate mixed-effect model, and all the models were adjusted for age, sex, body mass index, hypertension, diabetes, vaccine status, and COVID-19 waves.
Late CCP and late control groups had similar cytokine and chemokine levels through the follow-up periods (Table 1; Fig. S2 and S3).
DISCUSSION
While the neutralizing antibodies present in CCP are critical, this study offers another potential mechanism by which CCP reduces hospitalization from COVID-19. Overall, patients who received early CCP had lower levels of cytokines and chemokines at day 14 visit than a control group who received early SARS-CoV-2 seronegative plasma. Notably, IL-6 levels declined faster in the early CCP group during the first 2 weeks of follow-up after symptomatic COVID-19 infection.
IL-6 levels have been associated with COVID-19 morbidity and mortality due to increased inflammation and cytokine storms (22, 23). IL-6 plays an important role in a cascade of the immune system associated with generating a cytokine storm (22, 24). IL-6 neutralization is effective in reducing COVID-19 mortality (25), and IL-6 has been associated with post-COVID complications (11). Late CCP and late control groups showed similar cytokine levels during all follow-up visits, which further strengthens the importance of the timing of CCP. Given that CCP is an antiviral agent, one possible mechanism for the reduced IL-6 is a reduction in viral burden (26). Observing the significant decrease in the first 2 weeks compared to 3 months can potentially illustrate the role CCP treatment plays in reducing inflammation immediately after infection.
This study has both strengths and limitations. It is one of the largest outpatient studies that measures cytokine and chemokine levels for COVID-19, and data were collected over time, providing a trajectory before and after treatment. The study population is from a randomized controlled trial, which minimizes bias regarding who received CCP treatment or pre-pandemic control plasma. However, despite minimal differences observed between participants receiving late or early CCP or control plasma, there may be unmeasured confounding. Most of the study participants were also enrolled before the availability of vaccines, which may limit inferences about the current stage of the pandemic. In addition, cytokine measurements can be variable, and thus outlier results were excluded. There may be significant variability in cytokine measurements (27), but we used the same assay and lot number to reduce this variation. Finally, only the ability of CCP to decrease cytokines was evaluated, but other mechanisms of action may also be important. Further investigation of the mechanism of clinical benefit is needed.
The role of IL-6 in COVID-19 progression and long-term effects through increased inflammation is well established (11, 22, 28). This study shows that CCP transfusion early after SARS-CoV-2 infection is associated with reduced IL-6 levels.
ACKNOWLEDGMENTS
The authors gratefully acknowledge the plasma donors and study participants who generously gave their time and biological specimens to the CSSC-001 and CSSC-004 trials and the passionate study personnel who facilitated these studies.
This study was funded principally by the U.S. Department of Defense’s (DOD) Joint Program Executive Office for Chemical, Biological, Radiological and Nuclear Defense (JPEO-CBRND), in collaboration with the Defense Health Agency (DHA) (contract number: W911QY2090012) with additional support from Bloomberg Philanthropies, State of Maryland, the National Institutes of Health (NIH) National Institute of Allergy and Infectious Diseases (NIAID) 3R01AI152078-01S1 and 3R01AI120938-05S1, National Institute of Diabetes and Digestive and Kidney Diseases (R01DK131926), National Heart Lung and Blood Institute (1K23HL151826), National Institute on Drug Abuse (NIDA) F31DA054849, NIH National Center for Advancing Translational Sciences (NCATS) U24TR001609, Division of Intramural Research NIAID NIH, Mental Wellness Foundation, Moriah Fund, Octapharma, HealthNetwork Foundation, and the Shear Family Foundation. The study sponsors did not contribute to the study design, the collection, analysis, and interpretation of data, or the decision to submit this manuscript for publication. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funders.
Kelly Gebo, a consultant for the Aspen Institute and Teach for America, served as a non-paid member of the scientific advisory board for Pfizer and wrote COVID management guidelines for UpToDate which are outside the scope of this paper.
Sonya L. Heath serves on the Data Monitoring Committee for Pfizer COVID Therapeutics, which is outside of the scope of this paper.
Yuriko Fukuta: Nothing to disclose.
Xianming Zhu: Nothing to disclose.
Andrew Redd: Nothing to disclose.
Sheriza Baksh: Nothing to disclose.
Alison G. Abraham: Consultant for Implementation Group Inc., Hirslanden Klinik, Zurich CH, and Elsevier.
Feben Habtehyimer: Nothing to disclose.
David Shade: Nothing to disclose.
Jessica Ruff: Nothing to disclose.
Malathi Ram: Nothing to disclose.
Oliver Laeyendecker: Nothing to disclose.
Reinaldo E. Fernandez: Nothing to disclose.
Eshan U. Patel: Nothing to disclose.
Owen R. Baker: Nothing to disclose.
Shmuel Shoham served on a CCP guideline panel.
Edward R. Cachay received unrestricted research grants from Gilead and Merck paid to the Regents of the University of California. He also participated in an advisory board for Theratechnologies on an unrelated topic.
Judith S. Currier consulted for Merck and Company in 2021; currently not on any guidelines panel.
Jonathan M. Gerber: Nothing to disclose.
Thomas J. Gniadek is currently employed by Fenwal, Inc., a Fresenius Kabi Company.
Barry Meisenberg: Nothing to disclose.
Donald N. Forthal: Nothing to disclose.
Laura L. Hammitt: Received research funding to her institution outside the submitted work from AstraZeneca, Merck, and Pfizer.
Moises A. Huaman reports contracts from Gilead Sciences Inc., Insmed Inc., and AN2 Therapeutics Inc. to the University of Cincinnati outside the submitted work.
Adam C. Levine: Nothing to disclose.
Giselle S. Mosnaim receives current research grant support from GlaxoSmithKline, Novartis, and Sanofi-Regeneron, receives consulting fees from Novartis and Genentech, and has received past research grant support from Teva, Alk-Abello, and Genentech.
Bela Patel: Participation in COVID-19 trials and pulmonary arterial hypertension (PAH) trials; no disclosures relevant to CCP.
James H. Paxton: Research funding from MindRhythm, Inc.
Jay S. Raval, Consultant and Advisor with Sanofi Genzyme; Board of Directors Member with the American Society for Apheresis; no overlap with CCP.
Catherine G. Sutcliffe: Nothing to disclose.
Shweta Anjan: Nothing to disclose.
Seble Kassaye helped to produce educational materials related to HIV with Integritas Communications, LLC, and Vindico Medical Education, LLC.
Janis E. Blair: Nothing to disclose.
Amy L. Gawad: Nothing to disclose.
Sabra L. Klein: Nothing to disclose.
Andrew Pekosz: Nothing to disclose.
Arturo Casadevall serves on the scientific advisory board of SAB Therapeutics.
Evan M. Bloch reports personal fees and non-financial support from Terumo BCT, Abbott Laboratories, Tegus, and UpToDate outside of the submitted work. E.M.B. is a member of the United States Food and Drug Administration's (FDA) Blood Products Advisory Committee. Served on a CCP guideline panel.
Daniel Hanley reports personal fees from Neurelis, Neurotrope, and medicolegal consulting.
Aaron A.R. Tobian served on a CCP guideline panel.
David J. Sullivan is a founder and board member with stock options (macrolide for malaria). D.J.S. reports AliquantumRx, Hemex Health malaria diagnostics consulting, and royalties for malaria diagnostic test control standards to Alere, all outside the submitted work.
Kevin S. Oei: Nothing to disclose.
Matthew Abinante: Nothing to disclose.
Contributor Information
Aaron A. R. Tobian, Email: atobian1@jhmi.edu.
Benjamin M. Liu, Children's National Hospital, George Washington University, Washington, DC, USA
SUPPLEMENTAL MATERIAL
The following material is available online at https://doi.org/10.1128/spectrum.03286-23.
Fig. S1 to S3 and Table S1.
ASM does not own the copyrights to Supplemental Material that may be linked to, or accessed through, an article. The authors have granted ASM a non-exclusive, world-wide license to publish the Supplemental Material files. Please contact the corresponding author directly for reuse.
REFERENCES
- 1. Gustine JN, Jones D. 2021. Immunopathology of hyperinflammation in COVID-19. Am J Pathol 191:4–17. doi: 10.1016/j.ajpath.2020.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhu X, Gebo KA, Abraham AG, Habtehyimer F, Patel EU, Laeyendecker O, Gniadek TJ, Fernandez RE, Baker OR, Ram M, Cachay ER, Currier JS, Fukuta Y, Gerber JM, Heath SL, Meisenberg B, Huaman MA, Levine AC, Shenoy A, Anjan S, Blair JE, Cruser D, Forthal DN, Hammitt LL, Kassaye S, Mosnaim GS, Patel B, Paxton JH, Raval JS, Sutcliffe CG, Abinante M, Broderick P, Cluzet V, Cordisco ME, Greenblatt B, Petrini J, Rausch W, Shade D, Lane K, Gawad AL, Klein SL, Pekosz A, Shoham S, Casadevall A, Bloch EM, Hanley D, Sullivan DJ, Tobian AAR. 2023. Dynamics of inflammatory responses after SARS-Cov-2 infection by vaccination status: a prospective cohort study. Lancet Microbe 4:e692–e703. doi: 10.1016/S2666-5247(23)00171-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Scully EP, Schumock G, Fu M, Massaccesi G, Muschelli J, Betz J, Klein EY, West NE, Robinson M, Garibaldi BT, Bandeen-Roche K, Zeger S, Klein SL, Gupta A. 2021. Sex and gender differences in testing, hospital admission, clinical presentation, and drivers of severe outcomes from COVID-19. Open Forum Infect Dis 8:ofab448. doi: 10.1093/ofid/ofab448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bandopadhyay P, D’Rozario R, Lahiri A, Sarif J, Ray Y, Paul SR, Roy R, Maiti R, Chaudhuri K, Bagchi S, Maiti A, Perwez MM, Sarkar BS, Roy D, Chakraborty R, Vasudevan JS, Sharma S, Biswas D, Maiti C, Saha B, Bhattacharya P, Pandey R, Chatterjee S, Paul S, Ganguly D. 2021. Nature and dimensions of systemic hyperinflammation and its attenuation by convalescent plasma in severe COVID-19. J Infect Dis 224:565–574. doi: 10.1093/infdis/jiab010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pirofski LA, Casadevall A. 2020. Pathogenesis of COVID-19 from the perspective of the damage-response framework. mBio 11:e01175-20. doi: 10.1128/mBio.01175-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chi Y, Ge Y, Wu B, Zhang W, Wu T, Wen T, Liu J, Guo X, Huang C, Jiao Y, Zhu F, Zhu B, Cui L. 2020. Serum cytokine and chemokine profile in relation to the severity of coronavirus disease 2019 in China. J Infect Dis 222:746–754. doi: 10.1093/infdis/jiaa363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Noroozi R, Branicki W, Pyrc K, Łabaj PP, Pospiech E, Taheri M, Ghafouri-Fard S. 2020. Altered cytokine levels and immune responses in patients with SARS-Cov-2 infection and related conditions. Cytokine 133:155143. doi: 10.1016/j.cyto.2020.155143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Del Valle DM, Kim-Schulze S, Huang H-H, Beckmann ND, Nirenberg S, Wang B, Lavin Y, Swartz TH, Madduri D, Stock A, Marron TU, Xie H, Patel M, Tuballes K, Van Oekelen O, Rahman A, Kovatch P, Aberg JA, Schadt E, Jagannath S, Mazumdar M, Charney AW, Firpo-Betancourt A, Mendu DR, Jhang J, Reich D, Sigel K, Cordon-Cardo C, Feldmann M, Parekh S, Merad M, Gnjatic S. 2020. An inflammatory cytokine signature predicts COVID-19 severity and survival. Nat Med 26:1636–1643. doi: 10.1038/s41591-020-1051-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yongzhi X. 2021. COVID-19-associated cytokine storm syndrome and diagnostic principles: an old and new issue. Emerg Microbes Infect 10:266–276. doi: 10.1080/22221751.2021.1884503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Liu BM, Hill HR. 2020. Role of host immune and inflammatory responses in COVID-19 cases with underlying primary immunodeficiency: a review. J Interferon Cytokine Res 40:549–554. doi: 10.1089/jir.2020.0210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gebo KA, Heath SL, Fukuta Y, Zhu X, Baksh S, Abraham AG, Habtehyimer F, Shade D, Ruff J, Ram M, Laeyendecker O, Fernandez RE, Patel EU, Baker OR, Shoham S, Cachay ER, Currier JS, Gerber JM, Meisenberg B, Forthal DN, Hammitt LL, Huaman MA, Levine A, Mosnaim GS, Patel B, Paxton JH, Raval JS, Sutcliffe CG, Anjan S, Gniadek T, Kassaye S, Blair JE, Lane K, McBee NA, Gawad AL, Das P, Klein SL, Pekosz A, Casadevall A, Bloch EM, Hanley D, Tobian AAR, Sullivan DJ. 2023. Early treatment, inflammation and post-COVID conditions. mBio 11:e00618-23. doi: 10.1128/mbio.00618-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bonny TS, Patel EU, Zhu X, Bloch EM, Grabowski MK, Abraham AG, Littlefield K, Shrestha R, Benner SE, Laeyendecker O, Shoham S, Sullivan D, Quinn TC, Casadevall A, Pekosz A, Redd AD, Tobian AAR. 2021. Cytokine and Chemokine levels in Coronavirus disease 2019 Convalescent plasma. Open Forum Infect Dis 8:ofaa574. doi: 10.1093/ofid/ofaa574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sullivan DJ, Gebo KA, Shoham S, Bloch EM, Lau B, Shenoy AG, Mosnaim GS, Gniadek TJ, Fukuta Y, Patel B, Heath SL, Levine AC, Meisenberg BR, Spivak ES, Anjan S, Huaman MA, Blair JE, Currier JS, Paxton JH, Gerber JM, Petrini JR, Broderick PB, Rausch W, Cordisco M-E, Hammel J, Greenblatt B, Cluzet VC, Cruser D, Oei K, Abinante M, Hammitt LL, Sutcliffe CG, Forthal DN, Zand MS, Cachay ER, Raval JS, Kassaye SG, Foster EC, Roth M, Marshall CE, Yarava A, Lane K, McBee NA, Gawad AL, Karlen N, Singh A, Ford DE, Jabs DA, Appel LJ, Shade DM, Ehrhardt S, Baksh SN, Laeyendecker O, Pekosz A, Klein SL, Casadevall A, Tobian AAR, Hanley DF. 2022. Early outpatient treatment for COVID-19 with Convalescent plasma. N Engl J Med 386:1700–1711. doi: 10.1056/NEJMoa2119657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bloch EM, Shoham S, Casadevall A, Sachais BS, Shaz B, Winters JL, van Buskirk C, Grossman BJ, Joyner M, Henderson JP, et al. 2020. Deployment of convalescent plasma for the prevention and treatment of COVID-19. J Clin Invest 130:2757–2765. doi: 10.1172/JCI138745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tobian AAR, Cohn CS, Shaz BH. 2022. COVID-19 convalescent plasma. Blood 140:196–207. doi: 10.1182/blood.2021012248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Estcourt LJ, Cohn CS, Pagano MB, Iannizzi C, Kreuzberger N, Skoetz N, Allen ES, Bloch EM, Beaudoin G, Casadevall A, Devine DV, Foroutan F, Gniadek TJ, Goel R, Gorlin J, Grossman BJ, Joyner MJ, Metcalf RA, Raval JS, Rice TW, Shaz BH, Vassallo RR, Winters JL, Tobian AAR. 2022. Clinical practice guidelines from the association for the advancement of blood and biotherapies (AABB): COVID-19 convalescent plasma. Ann Intern Med 175:1310–1321. doi: 10.7326/M22-1079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Joyner MJ, Carter RE, Senefeld JW, Klassen SA, Mills JR, Johnson PW, Theel ES, Wiggins CC, Bruno KA, Klompas AM, et al. 2021. Convalescent plasma antibody levels and the risk of death from COVID-19. N Engl J Med 384:1015–1027. doi: 10.1056/NEJMoa2031893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Senefeld JW, Johnson PW, Kunze KL, Bloch EM, van Helmond N, Golafshar MA, Klassen SA, Klompas AM, Sexton MA, Diaz Soto JC, et al. 2021. Access to and safety of COVID-19 convalescent plasma in the United States expanded access program: a national registry study. PLoS Med 18:e1003872. doi: 10.1371/journal.pmed.1003872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Salazar E, Christensen PA, Graviss EA, Nguyen DT, Castillo B, Chen J, Lopez BV, Eagar TN, Yi X, Zhao P, Rogers J, Shehabeldin A, Joseph D, Leveque C, Olsen RJ, Bernard DW, Gollihar J, Musser JM. 2020. Treatment of coronavirus disease 2019 patients with convalescent plasma reveals a signal of significantly decreased mortality. Am J Pathol 190:2290–2303. doi: 10.1016/j.ajpath.2020.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Al-Riyami AZ, Estcourt L, Rahimi-Levene N, Bloch EM, Goel R, Tiberghien P, Thibert JB, Bruun MT, Devine DV, Gammon RR, Wendel S, Toungouz Nevessignsky M, Grubovic Rastvorceva RM, Oreh A, Romon I, van den Berg K, Kitazawa J, Patidar G, So-Osman C, Wood EM. 2022. Early and out-of-hospital use of COVID-19 Convalescent plasma: an international assessment of utilization and feasibility. Vox Sang 117:1202–1210. doi: 10.1111/vox.13347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Merad M, Blish CA, Sallusto F, Iwasaki A. 2022. The immunology and immunopathology of COVID-19. Science 375:1122–1127. doi: 10.1126/science.abm8108 [DOI] [PubMed] [Google Scholar]
- 22. Copaescu A, Smibert O, Gibson A, Phillips EJ, Trubiano JA. 2020. The role of IL-6 and other mediators in the cytokine storm associated with SARS-CoV-2 infection. J Allergy Clin Immunol 146:518–534. doi: 10.1016/j.jaci.2020.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Han H, Ma Q, Li C, Liu R, Zhao L, Wang W, Zhang P, Liu X, Gao G, Liu F, Jiang Y, Cheng X, Zhu C, Xia Y. 2020. Profiling serum cytokines in COVID-19 patients reveals IL-6 and IL-10 are disease severity predictors. Emerg Microbes Infect 9:1123–1130. doi: 10.1080/22221751.2020.1770129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jones SA, Jenkins BJ. 2018. Recent insights into targeting the IL-6 cytokine family in inflammatory diseases and cancer. Nat Rev Immunol 18:773–789. doi: 10.1038/s41577-018-0066-7 [DOI] [PubMed] [Google Scholar]
- 25. Gordon AC, Mouncey PR, Al-Beidh F, Rowan KM, Nichol AD, Arabi YM, Annane D, Beane A, van Bentum-Puijk W, Berry LR, et al. , REMAP-CAP Investigators . 2021. Interleukin-6 receptor antagonists in critically ill patients with COVID-19. N Engl J Med 384:1491–1502. doi: 10.1056/NEJMoa2100433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Marconato M, Abela IA, Hauser A, Schwarzmüller M, Katzensteiner R, Braun DL, Epp S, Audigé A, Weber J, Rusert P, Schindler E, Pasin C, West E, Böni J, Kufner V, Huber M, Zaheri M, Schmutz S, Frey BM, Kouyos RD, Günthard HF, Manz MG, Trkola A. 2022. Antibodies from convalescent plasma promote SARS-CoV-2 clearance in individuals with and without endogenous antibody response. J Clin Invest 132:e158190. doi: 10.1172/JCI158190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Liu BM, Martins TB, Peterson LK, Hill HR. 2021. Clinical significance of measuring serum cytokine levels as inflammatory biomarkers in adult and pediatric COVID-19 cases: a review. Cytokine 142:155478. doi: 10.1016/j.cyto.2021.155478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kappelmann N, Dantzer R, Khandaker GM. 2021. Interleukin-6 as potential mediator of long-term neuropsychiatric symptoms of COVID-19. Psychoneuroendocrinology 131:105295. doi: 10.1016/j.psyneuen.2021.105295 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 to S3 and Table S1.