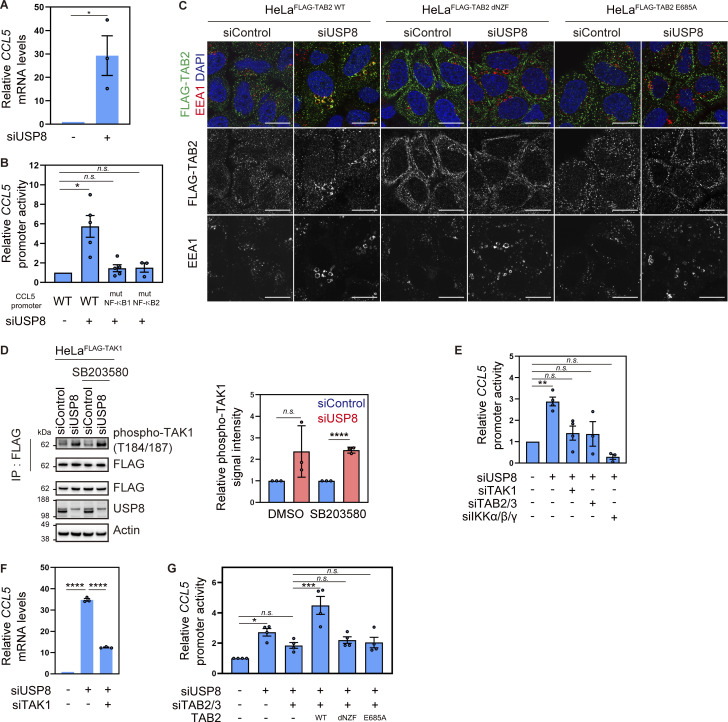
Figure 3.
Endosomal stress activates TAK1–NF-κB signaling by recruiting TAB2/3. (A) Total RNA from HeLa cells transfected with the indicated siRNAs was analyzed by qRT-PCR. CCL5 expression levels were normalized to actin mRNA levels, and expression levels in cells treated with control siRNA were set to 1. Individual values, mean, and SE of the mean of the relative mRNA levels are shown. Means ± SE were calculated from three biological replicates. *P < 0.05 (two-tailed Student’s t test). (B) HeLa cells transfected with the indicated siRNAs were analyzed by luciferase assay using vectors encoding either WT CCL5 promoter or mutants (mut) lacking NF-κB binding sites. The activities of the CCL5 promoter were normalized to those of the phosphoglycerate kinase (PGK) promoter, and the relative activities of the WT CCL5 promoter in cells treated with control siRNA were set to 1. Individual values, mean, and SE of the mean of the relative promoter activities are shown. Means ± SE were calculated from three and five biological replicates. *P < 0.05 (Kruskal–Wallis and Dunn’s tests). (C) HeLa cells stably expressing FLAG-TAB2 WT, dNZF, and E685A were transfected with the indicated siRNAs. Cells were immunostained with the indicated antibodies and DAPI. Scale bar, 20 μm. (D) HeLa cells stably expressing FLAG-TAK1 were transfected with the indicated siRNAs and treated with or without SB203580. FLAG-TAK1 immunoprecipitated with anti-FLAG antibody and total cell lysates were immunoblotted with the indicated antibodies (left). Signal intensities of phospho-TAK1 were quantified and normalized to those of total immunoprecipitated FLAG-TAK1 (right). Relative intensities in cells treated with control siRNA were set to 1. Individual values, mean, and SD of the mean of relative intensities are shown. Means ± SD were calculated from three biological replicates. ****P < 0.0001 (two-tailed Student’s t test). (E) HeLa cells transfected with the indicated siRNAs were analyzed by luciferase assay using vectors encoding the WT CCL5 promoter. The activities of the CCL5 promoter were normalized to those of the PGK promoter, and the relative activities of the WT CCL5 promoter in cells treated with control siRNA were set to 1. Individual values, mean, and SE of the mean of the relative promoter activities are shown. Means ± SE were calculated from three and four biological replicates. **P < 0.01 (one-way ANOVA with Dunnett’s test). (F) Total RNA from HeLa cells transfected with the indicated siRNAs was analyzed by qRT-PCR. CCL5 expression levels were normalized to actin mRNA levels, and expression levels in cells treated with control siRNA were set to 1. Individual values, mean, and SE of the mean of relative mRNA levels are shown. Means ± SE were calculated from three biological replicates. ****P < 0.0001 (one-way ANOVA with Dunnett’s test). (G) HeLa cells transfected with the indicated siRNAs and vectors to express the indicated proteins were analyzed by luciferase assay using vectors encoding the WT CCL5 promoter. The activities of the CCL5 promoter were normalized to those of the thymidine kinase (TK) promoter, and the relative activities of the WT CCL5 promoter in cells treated with control siRNA were set to 1. Individual values, mean, and SE of the mean of the relative promoter activities are shown. Means ± SE were calculated from four biological replicates. *P < 0.05, ***P < 0.001 (one-way ANOVA with Dunnett’s test). Source data are available for this figure: SourceData F3.