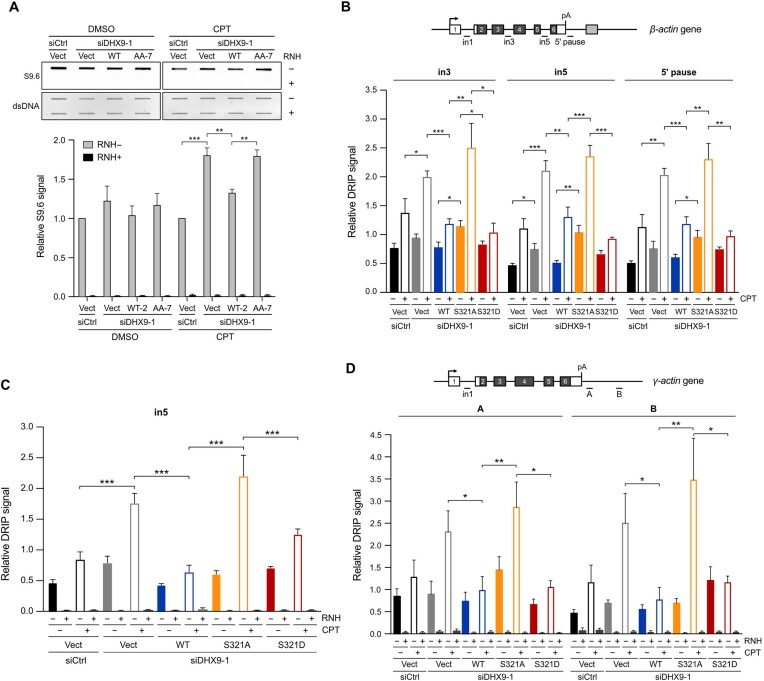
Figure 5.
Phospho-deficient DHX9 fails to suppress R-loops under genotoxic stress. (A) Disruption of DHX9 phosphorylation hindered R-loop suppression. HeLa stable lines were transfected with control siRNA or siDHX9 to silence endogenous DHX9, followed by the induction of SFB-DHX9WT, SFB-DHX9AA or the corresponding empty vector using doxycycline (200 ng/ml). The cell lines were treated with DMSO or CPT (1 μM) for 1 h. Genomic DNA extracted from treated cells was analyzed using slot blot assay with the S9.6 antibody. The relative S9.6 signal was quantified, and the data are presented as the mean ± SEM of three independent experiments. ** P < 0.01; *** P < 0.001. (B) Phosphomimetic DHX9 S321D suppressed the accumulation of R-loops in HeLa cells devoid of DHX9 after CPT treatment. Cells transfected with control or DHX9 siRNA were transiently expressed with SFB-DHX9 (WT, S321A and S321D), followed by CPT treatment (10 μM, 1 h). DNA/RNA hybrids purified using the S9.6 antibody were analyzed by RT-qPCR for the abundance of R-loops over β−actin gene. Values relative to in1 in each condition were plotted as the mean ± SEM of three experiments. Statistical significance was assessed by the Student's t-test (two-sided). * P < 0.05; ** P < 0.01; *** P < 0.001. (C) R-loop levels at the in5 of β−actin gene were suppressed by RNH. Experiments followed the protocol from (B) with the addition of RNH treatment to confirm that the increased DRIP signal was derived from R-loops. Values relative to in1 under each condition were plotted as the mean ± SEM of three experiments. Statistical significance was assessed by the Student's t-test (two-sided). *** P < 0.001. (D) Phosphomimetic S321D reduced R-loop accumulation over the γ-actin gene. Experiments were repeated as in (B), and RNH treatment was incorporated. Values relative to in1 in each condition were plotted as the mean ± SEM of three experiments. Statistical significance was assessed by the Student's t-test (two-sided). * P < 0.05; ** P < 0.01.