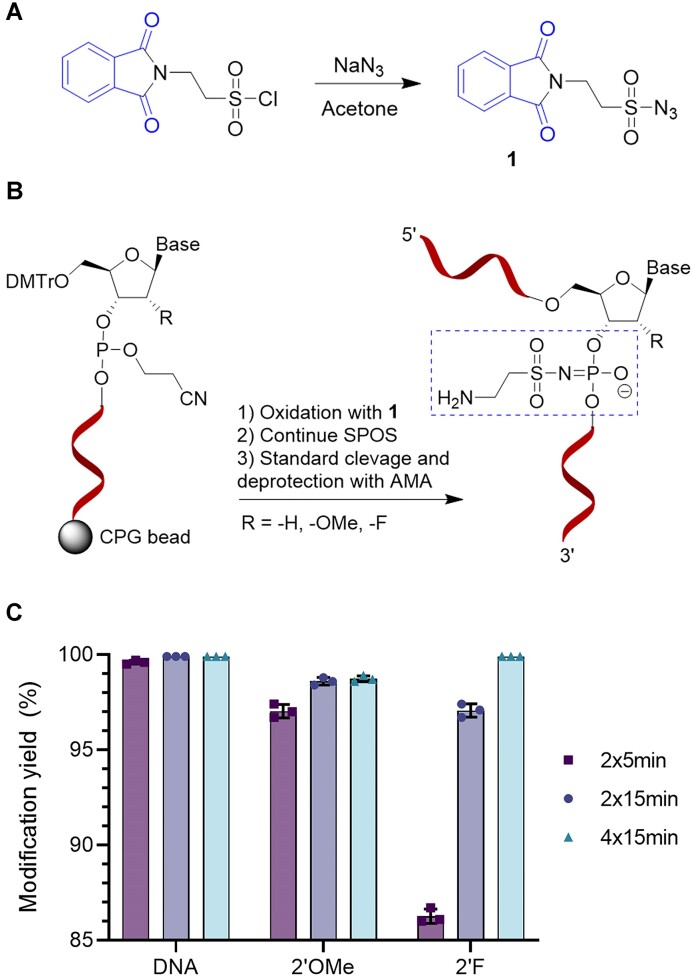
Figure 1.
Synthesis and reactivity of compound 1. (A) Synthesis of 1 is performed in a single step from 2-phthalimidoethanesulfonyl chloride. (B) Internal amino-modification of ONs is done by replacing the regular oxidation step for treatment with 1 during SPOS. (C) Modification yield was evaluated by HPLC analysis after SPOS of a DNA, 2′OMe or 2′F modified 8-mer ON with a single amino-modification made by treatment with 1. Data represents mean and standard deviation (SD) of n = 3 replicated syntheses.