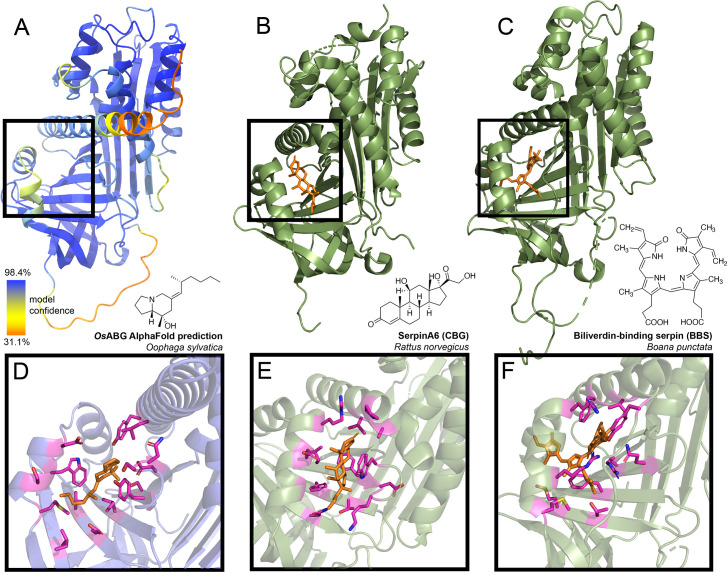
Figure 3. Predicted alkaloid-binding globulin (ABG) structure and binding pocket resembles that of other small molecule binding serpins.
(A) AlphaFold structure predicted with the protein sequence of the Oophaga sylvatica ABG, with color representing model confidence and predicted binding pocket based on molecular docking simulation indicated with a black box. (B) Crystal structure for rat SerpinA6/corticosteroid-binding globulin (CBG), with the cortisol molecule shown in orange (PDB# 2V95). (C) Crystal structure for tree frog Boana punctata biliverdin-binding serpin (BBS), with biliverdin shown in orange (PDB# 7RBW). (D) Close-up of predicted binding pocket of pumiliotoxin (PTX) in O. sylvatica ABG, with residues proximal to PTX highlighted in magenta. The structure of PTX is indicated on the top right. (E) Close-up of cortisol binding in CBG (PDB# 2V95), with proximal residues highlighted in magenta. Cortisol structure is displayed on the top right. (F) Close-up of biliverdin binding in BBS (PDB# 7RBW), with some proximal residues highlighted in magenta. Biliverdin structure is shown on the top right.