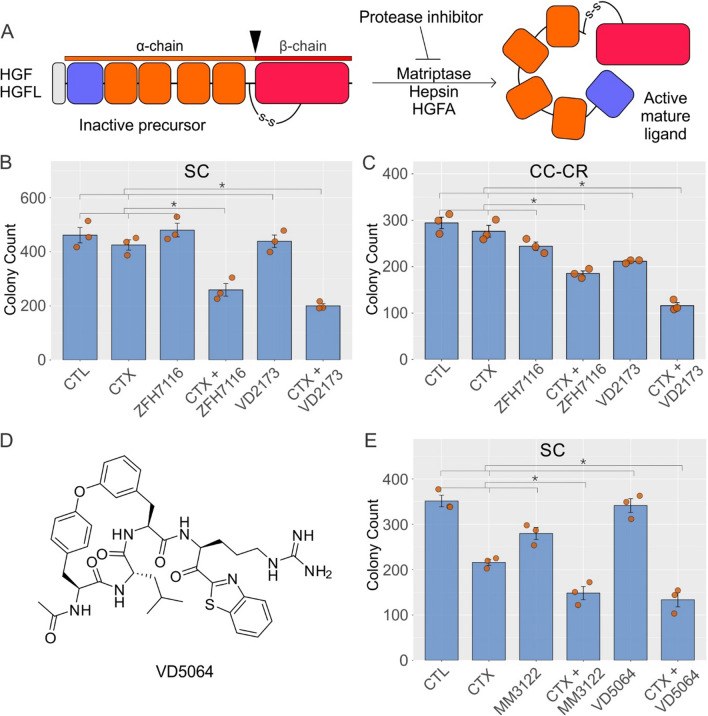
Fig. 1.
HGF/HGFL protease inhibitors overcome de novo and acquired modes of cetuximab resistance. A Domain organization and maturation of human HGF/HGFL. The 728/711-amino acid (aa) pre-propeptide consists of a 31/18-aa signal peptide (grey), a 87/85-aa PAN domain (purple), four 87–89-aa long Kringle domains (orange), and a 227/226-aa serine protease homology (SPH) domain (red). Ligand maturation requires proteolytic cleavage between α and β chains, as indicated by the arrowhead; inhibitors of these proteases (Matriptase, Hepsin, and HGFA) consequently limit availability of biologically active HGF/HGFL. Two thousand B HCA-7-derived spiky clone (SC) and C cetuximab-resistant cystic clone (CC-CR) were seeded in 3D in type I collagen and incubated with cetuximab (CTX, 3 μg/ml) alone or in combination with HGF/HGFL protease inhibitors ZFH7116 (50 μM) and VD2173 (50 μM) for 14–21 days. Colony counts are plotted as mean ± SEM; * indicates statistically significant differences (one-way ANOVA with Tukey's HSD post hoc test, p < 0.05). D Structure of the new triplex inhibitor VD5064. E Two thousand SC cells were seeded in 3D in type I collagen and incubated with CTX (3 μg/ml) alone or in combination with new generation of HGF/HGFL protease inhibitors MM3122 (25 μM) and VD5064 (25 μM) for 14–21 days. Colony counts are plotted as mean ± SEM; * indicates statistically significant differences (one-way ANOVA with Tukey’s HSD post hoc test, p < 0.05)