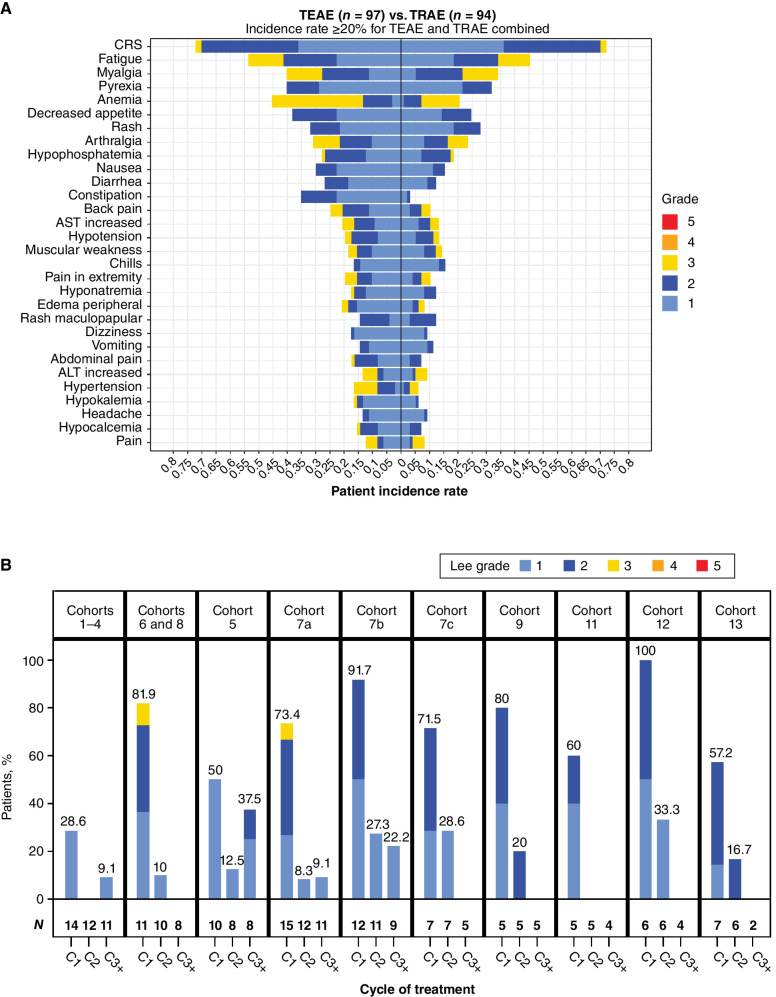
Figure 1.
A, Frequency and highest-grade AEs occurring in ≥20% of patients treated with xaluritamig across all cohorts, TEAE vs. TRAEs (defined by the investigator as having reasonable possibility of being caused by xaluritamig). B, Incidence and grade of CRS (32) by cycle and dose schedule. Cohort 10 was excluded (0.1–1.0 mg), as dosing schedule was adjusted for the remaining patients after initial patients with a 10-fold dose increase in 1 step experienced DLTs. ALT, alanine aminotransferase; AST, aspartate aminotransferase.