Abstract
Abdominal surgical site infections (SSIs) are infections that occur after abdominal surgery. They can be superficial, involving the skin tissue only, or more profound, involving deeper skin tissues including organs and implanted materials. Currently, SSIs are large global health problem with an incidence that varies significantly depending on the United Nations’ Human Development Index. The purpose of this review is to provide a practical update on the latest available literature on SSIs, focusing on causative pathogens and treatment with an overview of the ongoing studies of new therapeutic strategies.
Keywords: Surgical site infections, Multidrug resistance, Carbapenem-resistant Enterobacterales, Carbapenem-resistant Klebsiella, Abdominal post-operative complications, Post-surgical infections
Core Tip: Abdominal surgical site infections (SSIs) are infections that occur after abdominal surgery. The knowledge of the site of infection and the probability of antibiotic resistance is fundamental to initiating appropriate empirical antibiotic treatment. The purpose of this review is to provide a practical update on the latest available literature on SSIs, focusing on causative pathogens and treatment with an overview of the ongoing studies of new therapeutic strategies.
INTRODUCTION
Abdominal surgical site infections (SSIs) are infections that occur after abdominal surgery. They can be superficial, involving the skin tissue only, or more profound, involving deeper skin tissues including organs and implanted materials. Currently, SSIs are a large global health problem with an incidence that varies significantly depending on the United Nations’ Human Development Index (HDI). The incidence rates are observed at 9.4% in high HDI countries (HHDICs), 14.0% in middle HDI countries (MHDICs), and 23.2% in low HDI countries (LHDICs). Consensually, the antibiotic resistance incidence of the causative pathogen significantly varies in HHDICs (16.6%), MHDICs (19.8%), and LHDICs (35.9%). Intuitively, the highest incidence of abdominal SSIs is found in dirty surgery: HHDICs (17.8%), MHDICs (31.4%), and LHDICs (39.8%)[1]. Since approximately 234.2 [95% confidence interval (CI): 187.2-281.2] million major surgical procedures are carried out each year globally[2], with abdominal procedures (both major and minor surgery) the majority[3], abdominal SSIs are some of the largest concern worldwide. The United States Centers for Disease Control and Prevention (CDC) provide guidelines and resources to help end SSIs, along with assisting the public to understand and take measures to safeguard their health when possible[4]. Unfortunately, the CDC’s latest document, published in 2017, focuses only on SSI prevention[5].
The purpose of this review is to provide a practical update on the latest available literature on SSIs antimicrobial treatments.
CLASSIFICATION
In the 1960s, the National Academy of Sciences defined SSIs according to the type of surgery. Clean, clean-contaminated, contaminated, infected, or dirty surgery was the risk class. The SSI rate was 2.1%, 3.3%, 6.4%, and 7.1%, respectively[6]. However, a study by Neumayer et al[7] on general and vascular procedures reported that wound class was an independent predictor of SSI; odds ratios (ORs) were 1, 1.04, 1.7, and 1.5 for clean, clean-contaminated, contaminated, and infected, respectively. In the 2000s, the CDC and the National Healthcare Safety Network (NHSN) later classified SSIs according to the infection site, distinguishing superficial (infection of the skin and subcutaneous tissue), deep (fascia and muscle layers), or organ/space infections. Superficial SSIs occur within 30 d while deep SSIs occur within 30-90 d after the operative procedure, involving primary incision or secondary incision(s); their characteristics are reported in Table 1. An infection that involves both superficial and deep incision sites has to be classified as deep incisional SSI. Organ/space infections involve parts of the body being opened or manipulated during the operative procedure. If the organ/surface infection drains through the incision, it is classified as a deep SSI[8].
Table 1.
Definitions and clinical characteristics of surgical site infections according to Centers for Disease Control and Prevention and National Healthcare Safety Network criteria
|
Distinction
|
Superficial incisional SSI
|
Deep incisional SSI
|
Organ/space infections
|
|
| Localization | Subcutaneous tissue and/or skin | Fascial and muscle layers | Organ manipulated during surgery | |
| Timing | Within 30 d post-surgery | Within 30 or 90 d post-surgery/1 yr (implant in place) | ||
| Diagnosis at least one of the following | ||||
| Pain | Yes | Yes | Yes | |
| Swelling | Yes | Inconstant | Inconstant | |
| Erythema or heat | Yes | Inconstant | Inconstant | |
| Purulent drainage | Yes (superficial) | Yes (from deep incision) | No | |
| Wound dehiscence | Yes (superficial) | Yes | No | |
| Culture | Yes | Recommended | Recommended | |
| Abscess | No | Yes | Yes | |
| Fever (temperature > 38 °C) | Inconstant | Yes | Yes | |
SSI: Surgical site infection.
EPIDEMIOLOGY
The incidence of SSIs worldwide is highly variable, depending on the country and the type of surgery, but is approximately between 0.5% and 3%[9]. Abdominal surgery has a much higher rate of SSIs than other types of surgery, with an incidence of 15%-25%[10]. The main factors that determine this variability are attributed to the geographical region, the type of hospital, the type of intervention, the presence of surveillance institutions and how data is collected. Another crucial factor in the determinism of SSIs is the duration of surgery as demonstrated by Gillespie et al[11]. In a study conducted by the GlobalSurg collaborative group on patients undergoing an emergency appendectomy, there was a variable incidence of HHDICs, MHDICs, and LHDICs of 4.4%, 12%, and 14.8%, respectively[12]. The very high incidence of SSIs in low- and middle-income countries and Southeast Asia compared to the United States, Europe, and Australia can be related to several factors such as lack of standardized procedures, lack of epidemiological surveillance, lack of data interpretation, epidemiological data collected but not validated, poor-quality data records, and inefficient microbiological tools/poor laboratory capacity. According to a recent meta-analysis using the World Health Organization’s regions, Africa had the highest incidence, with Tanzania leading at 26%. The lowest incidence was found in the Western Pacific region within 0.6%[13]. There is significant variability in SSI surveillance practices resulting from differences in infection control resources among institutions, even in the United States[14]. Such hospitals using rigorous surveillance and broad data sources have reported higher SSIs rates compared with hospitals with lower surgical volumes that used fewer data to conduct surveillance and tend to have fewer SSIs rates. The accuracy of facility-reported SSIs rates is uncertain[15]. Data from the NHSN collected in the United States between 2006 and 2008 presented an overall SSIs rate of 1.9%[16]. Between 2008 and 2014, there was an overall 17% decrease in SSIs. A report from 2016 on the rates of hospital-acquired infections based on data from 2014 described an overall rate of 1.15%[17], with abdominal surgery-related SSIs as 50% of the overall SSIs. Furthermore, open surgery may significantly increase the incidence of SSIs if compared with laparoscopic surgery. A systematic review published in 2018 compared the incidence of SSIs in appendectomy performed worldwide. It reported that in HHDICs, the incidence rate of SSIs was 1.3%/3.8% for the open procedure and 0.8%/2.9% for the preventive laparoscopic technique. In LHDICs and MHDICs, the SSIs incidence rate was significantly higher with 17.9% reported for the open procedure and 8.8% for the laparoscopic approach[18]. A recent report from the European Centre for Disease Prevention and Control (ECDC) provided similar findings. The SSIs rates for open cholecystectomy vs laparoscopic and open vs laparoscopic colon surgery were 3.8% vs 1.5% and 9.5% vs 6.7%, respectively[19]. The overall surgery distribution of SSIs has changed both in high- and low-income countries over the past couple of decades, concerning antimicrobial prophylaxis[20]. The overall surgery distribution of pathogens associated with SSIs has varied over the years and the major organisms for abdominal surgery related to SSIs are Escherichia coli, Enterococcus faecalis, and Staphylococcus aureus[21]. By contrast, in developing countries, even in clean surgery, there is quite a high prevalence of Gram-negative bacilli such as Klebsiella species, E. coli and Pseudomonas aeruginosa[22]. The presence of Gram-negative bacilli is important because of high extended-spectrum beta-lactamase (ESβL) producer rates, and carbapenem-resistant Enterobacteriaceae (CRE) prevalence among these organisms that make antibiotic prophylaxis for clean or contaminated surgeries a challenge. The geographical distribution of the incidence of SSIS is depicted in Table 2. In Table 3, we synthesized data on the microbiology of SSIs subdivided for type of abdominal surgery, based on those reported by the ECDC annual epidemiological report for 2018-2020[19].
Table 2.
Geographical distribution of the surgical site infections’ incidence
|
Continent
|
Country
|
Period
|
SSIs incidence
|
Ref.
|
| Africa | Cameroon | 2013-2014 | Overall: 15.25% | [23] |
| Egypt | 2013-2017 | CSEC: 5.34% | [24] | |
| 2016-2018 | Overall: 2.3%, CSEC: 2.8% | [25] | ||
| Ethiopia | 2015 | Overall: 19.1% | [26] | |
| 2019 | Overall: 21.1%; ABDS: 49.06% | [27] | ||
| Ghana | 2017-2018 | Overall: 10%; APPY: 13.4%; GAST: 12.7%; HER: 5.9%; ABDS: 13.7% | [28] | |
| Kenya | 2015 | CSEC: 4% | [29] | |
| Morocco | 2018-2019 | Overall: 6.3% | [30] | |
| Rwanda | 2019-2020 | CSEC: 5.7% | [31] | |
| Sierra Leone | 2019-2020 | CSEC: 10.3%; HER: 1.2% | [32] | |
| 2021 | Overall: 11.5%; ABDS: 79.5% | [33] | ||
| South Africa | 2017 | APPY: 25% | [34] | |
| Tanzania | 2009-2010 | Overall: 26%; APPY: 15%; CHOL: 14.3%; XLAP: 27.9% | [35] | |
| 2018-2020 | CSEC: 14% | [36] | ||
| Tunisia | 2015-2016 | CSEC: 5% | [37,38] | |
| 2015 | APPY: 9.8%; CHOL: 1.1%; BILI: 13.6% | |||
| America | Brazil | 2008-2011 | Overall: 3.4% | [39] |
| 2008-2018 | BAR open: 3%; BAR VLP: 0.5% | [40] | ||
| Canada | 2015-2016 | CSEC: 5.9% | [41] | |
| 2015-2019 | COLO: 10.28%; BILI: 16.13% | [42] | ||
| Colombia | 2008-2010 | APPY: 3.9%; HYST: 5.5%; SPLE: 4.5%; CHOL: 3% | [43] | |
| 2022 | HER: 7.9%; CHOL: 8.3%; CSEC: 22.2% | [44] | ||
| Cuba | 2017-2018 | APPY: 13.8%; HER: 5.7% | [45] | |
| Ecuador | 2018 | CSEC: 1.35% | [46] | |
| Honduras | 2017-2018 | CSEC: 5.1% | [47] | |
| Mexico | 2011-2012 | Overall: 12.1%; COLO: 5.2%; APPY: 4.9%; CHOL: 0.8%; HER: 0.9% | [48] | |
| 2013-2015 | CHOL: 5.5% | [49] | ||
| Peru | 2005-2010 | APPY: 2.9%; CHOL: 2.8%; CSEC: 2.2% | [50] | |
| 2015-2018 | CSEC: 2.4% | [51] | ||
| 2019-2020 | CSEC: 0.88%; CHOL: 0.18%; HER: 0.38% | [52] | ||
| Uruguay | 2012-2013 | APPY: 3.2%; CHOL: 6.2%; COLO: 15.4% | [53] | |
| 2021 | CSEC: 1.74%; CHOL open: 1.85%; CHOL VLP: 0.23% | [54] | ||
| Venezuela | 2019-2021 | Overall: 9.7%; APPY: 10.42%; BILI 3.79% | [55] | |
| United States | 2011-2014 | Overall: 0.9%; COLO: 3.99%-9.47%; CHOLO: 0.23%-1.72%; HER: 0.74%-5.25%; REC: 3.47%-26.67%; SB: 3.44%-6.75% | [16,17,56] | |
| 2015-2019 | COLO: 6.82%; BILI: 12.72% | [42] | ||
| 2016-2017 | CHOL: 0.96% | [57] | ||
| Asia | China | 2020 | ABDS: 2.9%; COLO 7.1% | [58,59] |
| 2018 | GAST: 5.2% | [60] | ||
| 2017-2020 | CSEC: 23.30% | [61] | ||
| India | 2011-2017 | Appendix: 35.3% | [62] | |
| 2016 | CSEC: 10.3% | [63] | ||
| 2005-2011 | XLAP: 6%; HER: 3.8% | [64] | ||
| Iran | 2018 | Overall: 0.29% | [65] | |
| 2021 | Overall: 5.2% surveillance | [66] | ||
| Japan | 2008-2010 | COLO: 15%; REC: 17.8% | [67,68] | |
| 2009-2019 | APPY: VLP 4.19%, OPEN 6.60% | [69] | ||
| CHOL: VLP 1.91%, OPEN 7.42% | ||||
| SB: VLP 8%, OPEN 15% | ||||
| COLO: VLP 7.27%, OPEN 15.5% | ||||
| REC: VLP 11.3%, OPEN 8.8% | ||||
| Kuwait | 2016 | SB: 6.5%; GAST: 0.7% | [70] | |
| Nepal | 2019 | CSEC: 8.54% | [71] | |
| Pakistan | 2014-2019 | BILI: 40% | [72] | |
| 2016-2017 | APPY: 32.7%; CHOLO: 20.7%; HER: 37.6% | [73] | ||
| Philippines | 2018-2019 | Overall: 9.7% | [74] | |
| Republic of Korea | 2008-2012 | GAST: 3.12% | [75] | |
| Saudi Arabia | 2016 | Overall: 16.3%; open surgery: 34.8%; VLP surgery: 3.5% | [76] | |
| Taiwan | 2021 | Overall: 4.0%; regional hospital: 4.7% medical center | [77] | |
| Thailand | 2007-2016 | Overall: 2.98% | [78] | |
| Turkey | 2005-2011 | CHOL: 1.3%; COLO: 11.4%; CSEC: 3%; GAST: 4.3%; HYST: 3.1%; SPLE: 5%; XLAP: 2.6% | [79] | |
| United Arab Emirates | 2016-2017 | CSEC: 1.4% | [80] | |
| Europe | Austria | 2018-2020 | CHOL: 0.4%; COLO: 3.6%; CSEC: 0.5% | [19] |
| England | 2017-2022 | HYST: 1.7%; BILI: 15.4%; CHOL: 9.7%; GAST: 1.9%; COLO: 8.6% | [81] | |
| Estonia | 2018-2020 | CSEC: 2.0% | [19] | |
| France | 2018-2020 | CHOL: 0.7%; CSEC: 1.7% | [19] | |
| Germany | 2018-2020 | CHOL: 0.9%; COLO: 8.9%; CSEC: 0.6% | [19] | |
| Hungary | 2018-2020 | CHOL: 1.1%; COLO: 10.4%; CSEC: 1.3% | [19] | |
| Italy | 2018-2020 | CHOL: 0.7%; COLO: 5.9%; CSEC: 0.7% | [19] | |
| Lithuania | 2018-2020 | CHOL: 0.2%; COLO: 10.6%; CSEC: 0.6% | [19] | |
| Malta | 2018-2020 | COLO: 26.8% | [19] | |
| Netherlands | 2018-2020 | CHOL: 2.6%; COLO: 16.1%; CSEC: 1.5% | [19] | |
| Norway | 2018-2020 | CHOL: 2.8%; COLO: 11.7%; CSEC: 3.6% | [19] | |
| Portugal | 2018-2020 | CHOL: 2.3%; COLO: 14.5%; CSEC: 1.6% | [19] | |
| Slovakia | 2018-2020 | CHOL: 2.9% | [19] | |
| Spain | 2016 | COLO: 10.6% | [82] | |
| 2013-2016 | REC: 11.9% | [83] | ||
| 2009-2016 | CHOL: 1.96% | [84] | ||
| Switzerland | 2017-2018 | APPY: 3.1%; CHOL: 2.2%; HER: 0.9%; COLO: 13.5%; REC: 17.7%; GAST: 3.1%; CSEC: 1.8% | [85] | |
| Oceania | Australia | 2002-2013 | Overall: 2.8% | [86] |
ABDS: Abdominal surgery (miscellany); APPY: Appendix surgery; BAR: Bariatric surgery; BILI: Bile duct, liver or pancreatic surgery; CHOL: Gallbladder surgery; COLO: Colon surgery; CSEC: Caesarean section; GAST: Gastric surgery; HER: Herniorrhaphy; HYST: Abdominal hysterectomy; REC: Rectal surgery; SB: Small bowel surgery; SPLE: Spleen surgery; SSI: Surgical site infection; XLAP: Exploratory laparotomy.
Table 3.
Microorganisms distributions for different type of abdominal surgery
| Microorganism |
Type of surgery
|
||||
|
Laparoscopic CHOL
|
Open CHOL
|
Laparoscopic COLO
|
Open COLO
|
CSEC
|
|
| Gram-positive cocci | 52.9 | 39 | 34.8 | 70.4 | 78.8 |
| Staphylococcus aureus | 23.9 | 7.6 | 4.3 | 25 | 38.4 |
| Coagulase-negative staphylococci | 9.2 | 6.2 | 2.2 | 27 | 22.6 |
| Enterococcus species | 11.1 | 18.9 | 24.5 | 9.3 | 5.6 |
| Streptococcus species | 4.8 | 1.9 | 2.3 | 3.7 | 5.7 |
| Other Gram-positive cocci | 3.9 | 4.4 | 1.4 | 5.4 | 6.5 |
| Gram-positive bacilli | 1.5 | 0.8 | 0.1 | 2 | 2.7 |
| Gram-negative bacilli Enterobacterales | 27.5 | 44.4 | 48.7 | 18.3 | 10.6 |
| Escherichia coli | 13.2 | 21.7 | 30.4 | 6.2 | 3.5 |
| Citrobacter species | 0.5 | 2.4 | 1.1 | 0.7 | 0.4 |
| Enterobacter species | 2.3 | 5.6 | 5.5 | 3.3 | 2.8 |
| Klebsiella species | 5 | 9.8 | 6.2 | 2.1 | 1.4 |
| Proteus species | 3.5 | 2.1 | 2.1 | 3.8 | 1.3 |
| Serratia species | 1.1 | 0.3 | 0.9 | 1 | 0.8 |
| Other Enterobacteriaceae | 2.1 | 2.5 | 2.5 | 1.1 | 0.4 |
| Gram-negative nonfermentative bacilli | 4.2 | 2.1 | 6 | 3.8 | 3.3 |
| Acinetobacter species | 0.5 | 0.3 | 0.4 | 0.6 | 0.6 |
| Hemophilus species | 0.2 | 0.1 | 0 | 0 | 0.1 |
| Pseudomonas aeruginosa | 2.1 | 0.9 | 5.3 | 2.9 | 1.6 |
| Pseudomonadaceae family, other | 0.2 | 0 | 0 | 0.2 | 0.6 |
| Stenotrophomonas maltophilia | 0 | 0.3 | 0.1 | 0.1 | 0.3 |
| Other Gram-negative nonfermentative bacilli | 1.4 | 0.5 | 0.2 | 0.1 | 0 |
| Anaerobes | 12 | 8.7 | 6.3 | 4.8 | 3.8 |
| Bacteroides species | 1.8 | 1.2 | 4.4 | 0.2 | 0.1 |
| Other anaerobes | 10.2 | 7.5 | 2 | 4.6 | 3.7 |
| Other bacteria | 1.8 | 3.5 | 1 | 0.4 | 0.6 |
| Fungi, parasites | 0.2 | 1.5 | 3.2 | 0.3 | 0.1 |
| Candida species | 0.2 | 1.5 | 3.2 | 0.3 | 0.1 |
| Other fungi or parasites | 0 | 0 | 0 | 0 | 0 |
Data obtained from the ECDC’s Annual Epidemiological Report for 2018-2020 on surgical site infections. CHOL: Cholecystectomy; COLO: Colon surgery; CSEC: Caesarean section.
MICROBIOLOGY
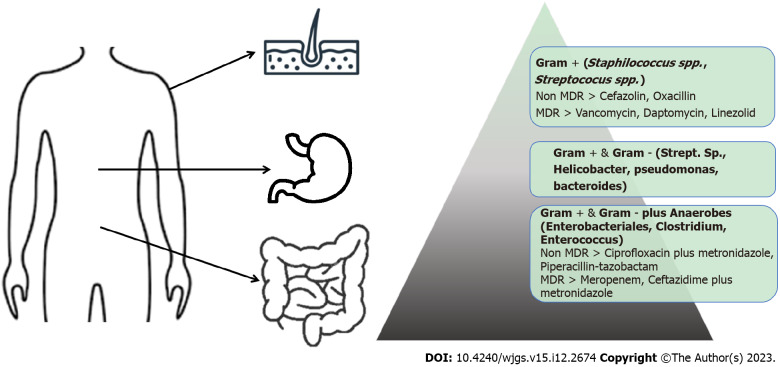
SSIs are one of the most common complications of abdominal surgery and are associated with increased morbidity, mortality, and costs[10]. SSIs can be defined as a wound infection with microorganisms within 30 d following a surgical procedure. They are caused by bacteria that enter the surgical site, originating from the patient’s endogenous flora or by nosocomial pathogens. The source of infection can be from the patient’s microbial flora, present on the skin and skin appendages, mucous membranes and the gastrointestinal tract, or insemination from a distant focus of infection. To prescribe antimicrobial therapy for an endogenous infection, knowledge of endogenous bacterial flora is crucial. The bacterial concentration increases along the gastrointestinal tract, with small numbers in the stomach and very high concentrations in the colon. This gradient is generated because the gastroduodenal tract is highly inhospitable for bacterial growth due to its pH, bile and pancreatic enzymes. Therefore, very few bacteria develop the ability to survive and multiply. The bacterial gradient is represented schematically in Figure 1. The stomach harbors only 101 bacteria per gram content. Increasing densities and bacterial diversities are found in the duodenum (103/g), jejunum (104/g), ileum (107/g), and colon (1012 bacteria/g)[86]. Besides a longitudinal gradient, there is also longitudinal diversity with Streptococcus, which is the most represented bacterium in the distal esophagus, duodenum, and jejunum. Helicobacter and Streptococcus are the dominant genera present in the stomach. The predominant phyla that inhabit the large intestine include Firmicutes and Bacteroidetes; the latter, together with Streptococcus, Enterobacteriaceae, Enterococcus, Clostridium, and Lactobacillus could be identified in stool[87]. The exogenous causes of infection are surgical personnel (surgeons and their teams), dirty clothing, potential “breakages” in aseptic techniques, and inadequate hand hygiene. Regarding the operating room, the causes of infection can be traced to the physical environment and the ventilation system, instrumentation, equipment, or other materials brought to the operating table. To reduce the risk of bacterial contamination preventative measures emphasize the importance of good patient preparation, aseptic practice, and attention to surgical technique. Antimicrobial prophylaxis is also indicated in specific circumstances. The most frequently isolated pathogens include Gram-positive cocci, such as S. aureus, enterococci and streptococci. Gram-negative bacilli, common pathogenic Enterobacteriaceae, including E. coli, Enterobacter species, Klebsiella species and Serratia marcescens are also found. P. aeruginosa and Acinetobacter baumannii are other common causes of Gram-negative infection[88]. Nosocomial pathogens, including Gram-negative and Gram-positive bacteria, are major causative microorganisms leading to epidemiological exposure[89]. The intensity and timing of the exposure, along with the virulence of the organism affect morbidity and mortality. Currently, novel threats are arising from multidrug-resistant (MDR) bacteria. An increasing number of SSIs result from MDR microorganisms. Among Gram-positive bacteria, we recognize methicillin-resistant S. aureus (MRSA) and vancomycin-resistant Enterococci[90]. Recently, a high rate of drug-resistant Gram-negative bacteria has become a major and global health concern[91,92]. The prevalence of Acinetobacter, Pseudomonas, and Gram-negative bacilli, which produce ESβL and carbapenemase, are increasing and related to higher rates of treatment failure[93,94]. Another key problem is the link between the SSIs and biofilm, where as many as 80% of these infections may involve a microbial biofilm. Recent studies suggest that biofilm-producing organisms play a significant role in persistent skin and soft tissue wound infections in the post-operative surgical patient population. SSIs associated with biomedical implants are notoriously difficult to eradicate using antibiotic regimens that would typically be effective against the same bacteria growing under planktonic conditions. This biofilm-mediated phenomenon is characterized as antimicrobial recalcitrance, which is associated with the survival of a subset of cells including “persister cells.” The ideal method to manage a biofilm-mediated surgical site wound infection is to prevent it from occurring in the first place through rational use of antibiotic prophylaxis, adequate skin anti-sepsis before surgery, and the use of innovative in situ irrigation procedures[95].
Figure 1.
Bacterial gradient with predominant bacteria. MDR: Multidrug resistance.
SOURCE CONTROL AND DRAINAGE
SSIs represent a serious problem for healthcare systems, especially in terms of length of hospital stay and cost. Over the years, many interventions have been proposed to reduce the SSIs rate. How an abdominal incision is closed has been largely investigated. A Cochrane meta-analysis reported there was no significant difference in terms of SSI rate and length of hospital stay when comparing continuous vs interrupted sutures for skin abdominal closure[96]. Moreover, the use of stitches with antimicrobial properties has been proven to reduce the SSIs rate in abdominal surgery. In particular, the use of triclosan-coated sutures is associated with a lower risk of SSIs[97]. Unfortunately, there is no evidence to prove the reduction of SSI with the use of intraoperative intraperitoneal irrigation and/or wound lavage with antibiotics. A topic that continues to be discussed and investigated in the literature[98,99]. Even wound irrigation before closure with saline or povidone solution has not proven to be valid in reducing SSIs[100]. Regarding mechanical devices, both single and dual-ring plastic wound protectors have proven to have a positive impact in preventing SSIs, with better results using the latter[101]. There is no concordance in the literature on the benefits related to the use of adhesive drapes (with or without antimicrobial properties) on a patient’s skin after surgical site cleaning. Also controversial is the role of subcutaneous drain placement before wound closure to reduce SSIs in high risk[102]. Regarding glove substitution during surgical procedures, changing gloves of all surgical teams at specific intervals especially in open surgery to avoid glove perforation or deterioration related to the duration of surgery appears to be beneficial[103]. Negative pressure wound therapy together with delayed abdominal closure (open abdomen technique) seems to be effective in preventing SSIs, especially in patients with a high risk of infection (highly contaminated peritoneum/wound)[104,105]. Normothermia, achieved with warming devices, is critical in reducing the rate of SSIs[106]. Perioperative oxygen supplementation is controversial and seems to be useless in reducing SSIs[107]. Understanding the time in which it can be useful to administer additional antibiotics intraoperatively is crucial to preventing SSIs, especially in patients undergoing urgent surgical procedures. Ultrasound-guided diagnostic and therapeutic drainage of fluid collections with the possibility of inserting a drain in a purulent cavity represents for surgeons a less-invasive bedside method to diagnose and solve a peritoneal pathological condition[108]. This useful tool represents an alternative to the classical surgical SSI source control gold standard consisting of debridement, removal of infected devices, drainage of collections, and decompression of the abdominal cavity. After an open abdomen technique, the timing to perform the gastrointestinal reconstruction and abdominal closure still widely debate in the literature. This suggests that further randomized clinical trials are needed to better define indications, timing, and techniques of the open abdomen technique in non-traumatic abdominal sepsis[109].
ANTIMICROBIAL MANAGEMENT
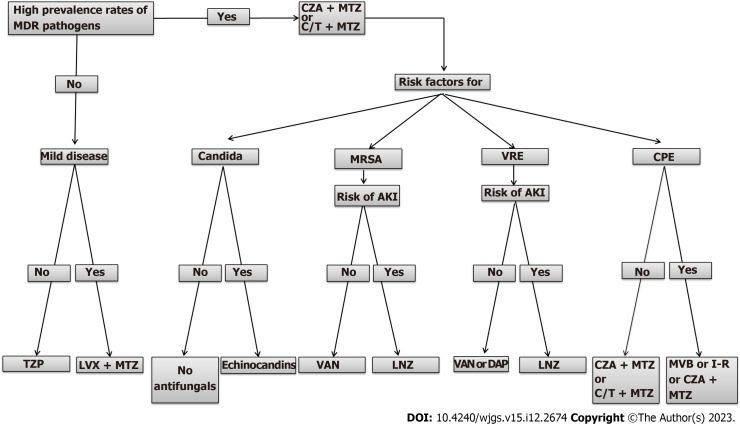
Antimicrobial treatment is one of the pillars of adequate management of SSIs following abdominal surgery, mainly in organ/space infections[93]. As mentioned earlier in this paper, SSIs after abdominal surgery are often polymicrobial, including, above all, Gram-negative and anaerobic bacteria[94,110]. An adequate empirical antimicrobial therapy should be administered as soon as possible. It is mainly based on: (1) The site of infection; (2) Disease severity, with the use of wider spectrum antibiotics for moderate/severe infections; and (3) Local epidemiology of MDR pathogens, with the use of wider spectrum antibiotics in centers with MDR high prevalence. Inadequate initial empiric antimicrobial treatment is an independent risk factor that negatively impacts patients’ outcomes. Several observations demonstrated that inadequate antimicrobial treatment is associated with an increased rate of morbidity and mortality. Moreover, an inadequate choice of initial treatments is associated with a longer hospital stay and higher costs of hospitalization compared with adequate antibiotic therapy[111,112]. The cornerstones of adequate antimicrobial therapy are proper etiological stratification, including local ecology and analysis of risk factors for MDR bacteria. This includes previous hospitalizations and antibiotic therapies (especially cephalosporins and quinolones) as well as stays in long-term care facilities and colonization with MDR bacteria. An evaluation of host characteristics, including hemodynamic status (presence or absence of signs of organ failure such as hypotension, oliguria, decreased mental alertness) and immunocompromised conditions (cancer or hematologic malignancy, human immunodeficiency virus, solid-organ transplant) that can influence the severity of abdominal SSIs is also relevant[100,113,114]. Every therapeutic choice must be framed within a broader antimicrobial stewardship strategy[115]. In non-critically ill patients without risk factors for MDR infections, a step-up approach can be reasonable. In these patients a single-agent therapy with broad-spectrum (e.g., levofloxacin, piperacillin/tazobactam, tigecycline) or a combination of metronidazole with cephalosporins (ceftriaxone and cefotaxime) or quinolones (ciprofloxacin and levofloxacin) can be used[116]. In addition, clinicians should be informed about the increased risk of antibiotic resistance among Gram-negative bacteria, mainly Enterobacteriaceae-producing EsβLs, observed in the last years and the extended use of quinolones that may be associated with the emergence of MDR bacteria[100,117]. Among, the new β-lactam and β-lactamase inhibitor (βLβI) combinations, ceftolozane/tazobactam (C/T) and ceftazidime/avibactam (CZA) have activity against Gram-negative bacteria with various antimicrobial resistance phenotypes, including EsβL producing strains. In the ASPECT-cIAI phase 3 studies, C/T plus metronidazole combination was non-inferior to meropenem (MER) regarding clinical cure in the microbiological intent-to-treat (83.0% vs 87.3%, respectively; difference: -4.2%; 95%CI: 8.91%-0.54%) and microbiologically evaluable (94.2% vs 94.7%, respectively; difference: -1.0%; 95%CI: -4.52% to 2.59%) populations. Among patients with infections due to EsβL producing strains, clinical cure rates were 95.8% and 88.5% in the C/T plus metronidazole and control groups, respectively[118]. Similarly, in the RECLAIM phase 3 studies, CZA plus metronidazole combination was non-inferior to MER regarding clinical cure in the microbiologically modified intention-to-treat (81.6% vs 85.1%, respectively; difference: -3.5%; 95%CI: -8.64% to 1.58%), in the modified intention-to-treat (82.5% vs 84.9%, respectively; difference: -2.4%; 95%CI: -6.90 to 2.10) and clinically evaluable (91.7% vs 92.5%, respectively; difference: -0.8%; 95%CI: -4.61 to 2.89) populations. A more aggressive approach should be considered in the clinical management of critically ill patients and those with risk factors for MDR bacteria. In these patients, carbapenems (MER and imipenem [IMI]/cilastatin [CIL]) or the above-mentioned βLβI combinations (plus metronidazole) represent the first line of treatment. However, the overuse of carbapenems has been associated with increased carbapenem resistance among Gram-negative bacteria, which has become a serious public health concern with worse clinical outcomes. Newly approved agents, MER/vaborbactam (MER/VAB) and IMI/CIL/relebactam (IMI/CIL/REL) are emerging options for the treatment of patients with abdominal SSIs, including those with infections due to MDR organisms (MDROs). MER/VAB is active against bacteria producing EsβL, KPC, and AmpC enzymes. In the TANGO-II phase 3 study, MER/VAB was associated with increased clinical cure and decreased mortality compared to the best available therapy (BAT) for the management of serious infections due to CRE. Overall, in the microbiologically modified intention-to-treat population, MER/VAB compared to BAT resulted in a higher rate of clinical cure at the end of therapy (65.6% vs 33.3%; P = 0.03) and the test-of-cure visit (59.4% vs 26.7%, respectively; P = 0.02). Furthermore, the 28-d all-cause mortality rate was 15.6% and 33.3% for MER/VAB vs BAT[119]. IMI/CIL/REL has a similar microbiological activity to MER/VAB. In the RESTORE-IMI-1 phase 3 study, IMI/CIL/REL was found to be an effective and well-tolerated treatment option for the management of serious infections due to CRE[120]. Another agent recently approved is eravacycline (EVC). It is a broad-spectrum antibiotic with activity against Gram-positive and Gram-negative MDROs, including CRE but not against P. aeruginosa. In IGNITE 1 and 4 phase 3 studies, EVC was compared to ertapenem and MER, respectively. Overall, EVC demonstrated non-inferiority to the comparators for the treatment of patients with complicated intra-abdominal infections[121,122]. In a post hoc analysis of IGNITE 1 and 4 studies, EVC showed a similar clinical outcome and microbiologic eradication rate compared to the MDR controls in bacteremic patients with primary complicated intra-abdominal infections[123]. Among new agents recently approved for the treatment of MDR Gram-negative cefiderocol (CFD) and plazomicin (PLZ) should be mentioned. CFD is a siderophore cephalosporin antibiotic with a broad spectrum of activity against Gram-negative bacteria, including MDROs such as CRE and carbapenem-resistant P. aeruginosa, and Acinetobacter baumannii[110,111]. In the CREDIBLE-CR phase 3 study, CFD has similar clinical and microbiological efficacy compared to BAT in the management of carbapenem-resistant Gram-negative infections[124]. PLZ, a new aminoglycoside, has broad-spectrum activity for Gram-positive and Gram-negative MDROs, including CRE[110,111]. In the CARE phase 3 study, the PLZ-based regimen was clinically and microbiologically effective in patients with serious infections due to CREs[125]. Antifungal agents should not be given empirically. In a randomized, double-blind, placebo-controlled trial assessing empirical antifungal treatment with micafungin (100 mg/d) in intensive care unit patients requiring surgery for intra-abdominal infection the incidence of invasive Candidiasis was 8.9% for placebo and 11.1% for micafungin group, with no difference in median time to invasive Candidiasis. Patients with a positive (1,3)-β-d-glucan (βDG) were 3.66 (95%CI: 1.01-13.29) times more likely to have invasive Candidiasis[126]. In cases of acute necrotizing pancreatitis, the use of antifungal agents seems to prevent fungal infection[127]. We have synthesized evidence in a pragmatic therapeutic algorithm for approaching the main empirical antimicrobial therapy for the management of SSIs (Figure 2).
Figure 2.
Empirical antimicrobial approach of abdominal post-surgical infections. CPE: Carbapenem-resistant Enterobacterales; C/T: Ceftolozane/tazobactam; CZA: Ceftazidime/avibactam; DAP: Daptomycin; I-R: Imipenem-relebactam; LVX: Levofloxacin; LNZ: Linezolid; MDR: Multidrug-resistance; MRSA: Methicillin-resistant Staphylococcus aureus; MTZ: Metronidazole; MVB: Meropenem-vaborbactam; TZP: Piperacillin-tazobactam; VAN: Vancomycin; VRE: Vancomycin-resistant Enterococci.
FUTURE PERSPECTIVES
On September 30, 2023, clinicaltrial.gov recorded 34 clinical studies in the field of pharmacological and physical strategies for the prevention of SSIs in abdominal surgery; 10 are in the recruiting phase. Two phase III, prospective, multinational, multicenter, randomized, controlled, two-arm, double-blind studies (NCT04411199 and NCT04233424) compared the use of a new formulation of extended-release of doxycycline (D-PLEX). D-PLEX is supplied as a sterile powder to be reconstituted to paste in the operating room and is intended for single administration. The non-active components of the extended-release antibiotic formulation are β tri-calcium polymer and a lipid matrix. It must be applied during the surgery at the final stage of incision closure. Falcon trial (NCT03700749) is a double-blind 2x2 factorial, stratified, multi-center randomized controlled trial where recruited participants will be randomly assigned to four arms receiving different combinations of skin preparation and sutures for wound closure: 2% alcoholic chlorhexidine for skin cleansing and non-coated suture (arm A); 2% alcoholic chlorhexidine for skin cleansing and triclosan coated suture (arm B); 10% aqueous povidone-iodine for skin cleansing and non-coated suture (arm C); and 10% aqueous povidone-iodine for skin cleansing and triclosan-coated suture (arm D). Preoperative antiseptic chlorhexidine-based alcohol has been established as the gold standard of care for clean contaminated wounds. Topical prophylaxis of the surgical wound with antibiotics is one of the most controversial measures proposed for SSIs prevention and the World Health Organization considers irrigation with antibiotics an unresolved issue. Some ongoing trials compare the use of topical antibiotics or their irrigation such as gemcitabine/clindamycin in the RINSE trial (NCT03945357) or amoxicillin-clavulanate (NCT04476212) vs saline irrigation. Closed incision negative pressure therapy (CINVt) is a new potential treatment strategy to reduce SSIs. This technique is based on the application of local negative pressure to the wound surface. The literature on its effectiveness is unclear. Two ongoing trials NCT04496180 and NCT04110353 compare the effectiveness of CINVt in reducing the incidence of SSIs vs simple standard dressing. The overview of ongoing trials shows that there is currently no introduction of new effective molecules in the treatment of abdominal post-surgical infections (Table 4). In fact, despite increased antibiotic resistance, pharmaceutical companies are hesitant to develop new antibiotics due to scientific, regulatory, and financial obstacles[128]. Li et al[129] in an observational cohort study, enrolling 2014 elderly patients who had elective surgery from 28 hospitals in China, developed and validated deep learning-based predictive models for post-operative infections in the elderly. The deep learning model predicted post-operative infections with an OR of 0.763 (95%CI: 0.681-0.844) with a sensitivity of 63.2% (95%CI: 46-78.2) and a specificity of 80.5% (95%CI: 76.6-84). Given the lack of new antibiotics deep learning models that incorporate risk factors for the prediction of abdominal post-surgical infections should be explored in future studies.
Table 4.
Ongoing trials
|
Study name
|
ClinicalTrial.gov identifier
|
Design
|
Status
|
Type of surgery
|
Intervention(s)
|
Country
|
| Iodine-Povidone Alcohol Compared to Chlorhexidine Alcohol as Preoperative Antiseptics in Major Abdominal Elective Clean Contaminated Surgery | NCT03859908 | Single blind RCT | Terminated | Elective surgery categorized as clean contaminated surgery | Drug: Iodine povacrylex/isopropyl alcohol | El Salvador |
| Drug: Chlorhexidine gluconate/isopropyl alcohol | ||||||
| Examination of the Effect of Skin Antisepsis with Pre-heated Povidone Iodine on Surgical Site Infections: A Quasi-Experimental Study | NCT04969302 | Single blind RCT | Completed | NS | Experimental: Povidone-iodine will heat to 37 °C using a gel warmer | Greece |
| Control: Povidone-iodine will heat to 20 °C using a gel warmer | ||||||
| Study to Assess the Safety & Efficacy of Oral Ciprodiazole Versus Currently Used Ciprofloxacin & Metronidazole (CIPRO-001) | NCT05863832 | Open label RCT | Recruiting | Pelvi-abdominal surgery | Experimental | Egypt |
| Ciprodiazole | ||||||
| Active comparator: Ciprofloxacin 500 mg | ||||||
| PVP Iodine vs Chlorhexidine in Alcohol for Disinfection of the Surgical Site (PICASSo) | NCT03685604 | Single blind RCT | Completed | Colorectal surgery, cholecystectomy, herniotomy, appendectomy and bariatric surgery | Active comparator: Braunoderm® | Switzerland |
| Comparator | ||||||
| Softasept® | ||||||
| Delafloxacin IV and OS Administration Compared to Best Available Therapy in Patients with Surgical Site Infections (DRESS) | NCT04042077 | Single blind RCT | Terminated | Abdominal surgery | Drug: Delafloxacin | Rome |
| Drug: Vancomycin | ||||||
| Drug: Linezolid | ||||||
| Drug: Piperacillin/tazobactam | ||||||
| Drug: Tigecycline | ||||||
| A Randomized, Blinded, Placebo and Standard of Care Controlled Efficacy, Safety, and Tolerability Study of up to 20 mL of DFA-02 in Patients Undergoing Abdominal Surgery | NCT01888367 | Triple blind RCT | Completed | Abdominal surgery | Drug: DFA-02 Antibiotic Gel | United States |
| Drug: DFA-02 Placebo Gel | ||||||
| Reduction of Postoperative Wound Infections by Antiseptica? (RECIPE) | NCT04055233 | Double blind RCT | Completed | Laparotomy for visceral surgery | Drug: Polihexanide; Serasept | Germany |
| Drug: NaCl; saline | ||||||
| Study of Chlorhexidine Gluconate as a Preoperative Antisepsis (CHG) | NCT01495117 | Quadruple blind RCT | Completed | Resection surgery (clean-contaminated open surgery) | Drug: Povidone-Iodine | Republic of Korea |
| Drug: Chlorhexidine gluconate | ||||||
| A Randomized Controlled Trial of 2% Chlorhexidine Gluconate Skin Preparation Cloths for the Prevention of Post-Operative Surgical Site Infections in Colorectal Patients | NCT02385708 | Open label RCT | Completed | Colorectal surgery | Drug: 2% chlorohexidine gluconate standard of care | United States |
| Drug: 2% chlorohexidine gluconate chin to toe | ||||||
| Effect of Peritoneal Lavage with Clindamycin-gentamicin Solution on Postoperative Colorectal Cancer Infection in Elective Surgery | NCT01378832 | Open label RCT | Completed | Colorectal surgery | Procedure: Intra-peritoneal antibiotic lavage | No location data |
| Collagen-Gentamicin Implant in the Treatment of Contaminated Surgical Abdominal Wounds - A Randomized Controlled Trial | NCT00977405 | Double blind RCT | Terminated | Abdominal surgery | Device: Collatamp gentamicin implant | Singapore |
| CLinical Evaluation of Adults UNdergoing Elective Surgery Utilizing Intraoperative Incisional Wound Irrigation: A Randomized Controlled Trial (CLEAN Wound) | NCT04548661 | Double blind RCT | Not yet recruiting | Laparotomy (clean-contaminated or contaminated incision). Laparoscopy (clean-contaminated or contaminated incision) | Procedure: Intraoperative incisional wound irrigation with povidone-iodine solution | Canada |
| Procedure: Intraoperative incisional wound irrigation with saline | ||||||
| Randomized Controlled Trial to Evaluate the Optimal Timing of Surgical Antimicrobial Prophylaxis | NCT01790529 | Quadruple blind RCT | Completed | Colorectal surgery | Procedure: Cefuroxime + metronidazole 75 to 30 min prior to skin incision | Switzerland |
| Procedure: Cefuroxime + metronidazole within 30 min prior to skin incision) | ||||||
| A Pilot Clinical Evaluation of the Antimicrobial Effectiveness of Topically Applied ZuraPrep™ | NCT02221232 | Open label pilot study | Terminated | NS | Drug: Chloraprep | United States |
| Drug: ZuraPrep | ||||||
| Drug: ZuraPrep Vehicle | ||||||
| ROSSINI 2 - Reduction of Surgical Site Infection Using Several Novel Interventions (ROSSINI 2) | NCT03838575 | Double blind RCT | Recruiting | Colorectal, hepatobiliary, upper GI, urological, vascular, or gynecological | Drug: 2% alcoholic chlorhexidine skin prep (SKIN PREP) | United Kingdom |
| Device: Iodophor Antimicrobial Incise Drapes (DRAPE) | ||||||
| Device: Gentamicin-impregnated implants/sponges (SPONGE) | ||||||
| Other: None (control) | ||||||
| D-PLEX 311: Safety and Efficacy of D-PLEX in the Prevention of Post Abdominal Surgery Incisional Infection (SHIELD I) | NCT04233424 | Triple blind RCT | Completed | Elective colorectal surgery | Drug: D-PLEX (new formulation of extended release of doxycycline) | United States |
| Other: SoC | ||||||
| D-PLEX 312 - Safety and Efficacy of D-PLEX in the Prevention of Post Abdominal Surgery Incisional Infection (SHIELD II) | NCT04411199 | Triple blind RCT | Recruiting | Elective colorectal surgery | Drug: D-PLEX + SoC | United States, Hungary, Serbia, Poland, Israel |
| Other: SoC | ||||||
| Abdomen Closure Using Triclosan Coated Absorbable Suture vs Uncoated Sutures of the Same Base Material | NCT01620294 | Double blind RCT | Completed | Elective colorectal surgery | Procedure: Abdominal wall closure | Hungary |
| Procedure: Surgical site infection | ||||||
| Prophylaxis of Surgical Wound Infection with Topical Antibiotics | NCT04476212 | Triple blind RCT | Recruiting | Elective abdominal wall surgery | Drug: Amoxicillin-clavulanate for topical prophylaxis |
Spain |
| Elective and emergency colorectal surgery | No intervention: Control | |||||
| D-PLEX 310: Safety and Efficacy of D-PLEX in the Prevention of Post Abdominal Surgery Incisional Infection | NCT03633123 | Single blind RCT | Completed | Elective colorectal surgery | Drug: D_PLEX | Israel |
| Other: SoC | ||||||
| Antibiotic Prophylaxis in the Prevention of Surgical Site Infections After Selected Urgent Abdominal Surgical Procedures | NCT01524081 | Double blind RCT | Completed | Emergent surgery for: Acute appendicitis/perforated gastric or duodenal ulcer/small bowel obstruction | Drug: Metronidazole, cefuroxime | Czech Republic |
| Drug: Amoxicillin (+ clavulanic acid) and fluconazole | ||||||
| Drug: Placebo | ||||||
| Drug: Placebo | ||||||
| Study the Efficacy of Topical Antibiotherapy in the Prophylaxis of Incisional Surgical Infection in Colorectal Surgery (PROTOP) | NCT03574090 | Triple blind RCT | Completed | Colorectal surgery | Drug: Amoxicillin clavulanate | Spain |
| Drug: Physiological saline | ||||||
| Parenteral Antibiotics Compared to Combination of Oral and Parenteral Antibiotics in Colorectal Surgery Prophylaxis (ORALEV) | NCT02505581 | Quadruple blind RCT | Completed | Colorectal surgery | Drug: Extra dosage - cefuroxime (750 mg) intravenous | Spain |
| Drug: Ciprofloxacin 750 mg oral | ||||||
| Drug: Metronidazole 250 mg oral | ||||||
| Drug: Cefuroxime 1.5 g intravenous | ||||||
| Drug: Metronidazole 1 g intravenous | ||||||
| Impact of Triclosan-coated Suture on Surgical Site Infection After Colorectal Surgery | NCT01869257 | Single blind RCT | Completed | Colorectal surgery | Device: Triclosan coated suture | Italy |
| Device: Regular suture | ||||||
| Intravenous Versus Combined Oral and Intravenous Antimicrobial Prophylaxis for the Prevention of Surgical Site Infection in Elective Colorectal Surgery (COMBINE) | NCT02618720 | Double blind RCT | Completed | Elective colorectal surgery | Drug: Ornidazole | France |
| Drug: Placebo | ||||||
| Prophylactic Effect Preoperative Antibiotics with Mechanical Bowel Preparation in SSIs | NCT03856671 | Open label RCT | Completed | Laparoscopic colorectal surgery | Drug: Neomycin, metronidazole | China |
| Frequency of Surgical Site Infection in Abdominal Hernia with Gentamycin Spray on Mesh Versus no Spray | NCT04164524 | Case-Control trial | Completed | Elective surgery; Para umbilical hernia, umbilical and epigastric hernia, | Drug: Gentamycin 160 mg spray applied over the mesh | Pakistan |
| Antibiotic Instillation in Acute Complex Appendicitis for Prevention of Deep Space Surgical Site Infections | NCT05470517 | Single blind RCT | Recruiting | Appendectomy | Drug: Ceftriaxone | United States |
| Procedure: Intra-peritoneal fluid aspiration | ||||||
| Prophylaxis of Surgical Wound Infection in Incisional Hernia Repair With Topical Antibiotics (PROTOP-PAR) | NCT05508152 | Triple blind RCT | Recruiting | Elective surgical procedure due to an abdominal wall incisional hernia | Drug: Wound irrigation with amoxicillin-clavulanate in saline solution | Spain |
| Drug: Wound irrigation with a saline solution | ||||||
| Orally Administered Trimethoprim-sulfamethoxazole and Metronidazole as Prophylaxis of Infection Following Elective Colorectal Surgery | NCT00613769 | Triple blind RCT | Completed | Colorectal surgery | Drug: Trimethoprim-sulfamethoxazole + metronidazole | Sweden |
| Drug: Cefuroxime and metronidazole | ||||||
| The Effect of Intraoperative Peritoneal Lavage With Super-Oxidized Solution on Surgical Site Infections and Mortality in Patients With Secondary Peritonitis: A Randomized Controlled Trial | NCT05050253 | Open label RCT | Recruiting | Emergency abdominal surgery by laparotomy | Device: Super-oxidized solution | Switzerland |
| Device: Ringer’s solution | ||||||
| Reducing INfection at the Surgical SitE With Antibiotic Irrigation During Ventral Hernia Repair (RINSE Trial) | NCT03945357 | Open label RCT | Completed | Elective, open ventral hernia repair | Drug: Gemcitabine/clindamycin | United States |
| Drug: Normal saline | ||||||
| Preoperative Oral Antibiotics With vs Without Mechanical Bowel Preparation to Reduce Surgical Site Infections Following Colonic Resection: an International Randomized Controlled Trial. (ORALEV2) | NCT04161599 | Single blind RCT | Recruiting | Colectomy | Drug: Cefuroxime (750 mg) intravenous | China |
| Drug: Cefuroxime 750 mg oral | Italy | |||||
| Drug: Metronidazole 250 mg oral tablet | Spain | |||||
| Drug: Metronidazole 1 g intravenous | Russia | |||||
| Drug: Cefuroxime 1.5 g intravenous | Greece | |||||
| Drug: Sodium picosulfate, light magnesium oxide, anhydrous citric acid 10 mg/3.5 g/10.97 g oral | United Kingdom | |||||
| Standard Versus Pre-emptive Antibiotic Treatment to Reduce the Rate of Infectious Outcomes After Whipple’s Procedure (SPARROW): a Multicenter, Randomized Controlled Trial | NCT05784311 | Open label RCT | No yet recruiting | Elective pancreatoduodenectomy | Drug: Cefuroxime | Netherlands |
| Drug: Metronidazole |
NS: Not specified; RCT: Randomized controlled trial; SoC: Standard of care.
CONCLUSION
Abdominal infections are some of the most common healthcare-associated problems, occurring 15%-25% after surgical procedures. Rapid clinical diagnosis and empirical antimicrobial therapy are essential. According to the CDC and NHSN; after a clinical diagnosis of SSI is made, adequate empirical antimicrobial therapy should be administered as soon as possible. The choice of antimicrobial therapy is based on three pillars: The site of infection, the disease severity, and the local epidemiology of MDROs. Few antibiotics are now available to treat such infections, and thus should not be used for mild infections in centers where the incidence of MDROs is low. This strategy is essential to prevent bacterial resistance. This review was written to provide a practical update on the latest available literature on SSIs and antimicrobial treatments. Due to the decreasing of number of new antibiotics development and improvement artificial intelligence should be explored for the prediction of abdominal post-surgical infections in future studies.
Footnotes
Conflict-of-interest statement: The authors have no conflicts of interest to declare.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: July 29, 2023
First decision: September 14, 2023
Article in press: November 21, 2023
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: He YH, China; Li J, China S-Editor: Wang JJ L-Editor: Filipodia P-Editor: Wang JJ
Contributor Information
Marco Fiore, Department of Women, Child and General and Specialized Surgery, University of Campania “Luigi Vanvitelli,” Naples 80138, Italyy. marco.fiore@unicampania.it.
Antonio Corrente, Department of Women, Child and General and Specialized Surgery, University of Campania “Luigi Vanvitelli,” Naples 80138, Italy.
Sveva Di Franco, Department of Women, Child and General and Specialized Surgery, University of Campania “Luigi Vanvitelli,” Naples 80138, Italy.
Aniello Alfieri, Department of Women, Child and General and Specialized Surgery, University of Campania “Luigi Vanvitelli,” Naples 80138, Italy.
Maria Caterina Pace, Department of Women, Child and General and Specialized Surgery, University of Campania “Luigi Vanvitelli,” Naples 80138, Italy.
Francesca Martora, Unit of Virology and Microbiology, “Umberto I” Hospital, Nocera Inferiore 84018, Italy.
Stephen Petrou, Department of Emergency Medicine, University of California San Francisco, San Francisco, CA 94143, United States.
Claudio Mauriello, Department of General Surgery, “Santa Maria delle Grazie” Hospital, Pozzuoli 80078, Italy.
Sebastiano Leone, Division of Infectious Diseases, “San Giuseppe Moscati” Hospital, Avellino 83100, Italy.
References
- 1.GlobalSurg Collaborative. Surgical site infection after gastrointestinal surgery in high-income, middle-income, and low-income countries: a prospective, international, multicentre cohort study. Lancet Infect Dis. 2018;18:516–525. doi: 10.1016/S1473-3099(18)30101-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weiser TG, Regenbogen SE, Thompson KD, Haynes AB, Lipsitz SR, Berry WR, Gawande AA. An estimation of the global volume of surgery: a modelling strategy based on available data. Lancet. 2008;372:139–144. doi: 10.1016/S0140-6736(08)60878-8. [DOI] [PubMed] [Google Scholar]
- 3.Abbott TEF, Fowler AJ, Dobbs TD, Harrison EM, Gillies MA, Pearse RM. Frequency of surgical treatment and related hospital procedures in the UK: a national ecological study using hospital episode statistics. Br J Anaesth. 2017;119:249–257. doi: 10.1093/bja/aex137. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. Division of Healthcare Quality Promotion (DHQP). [cited 30 April 2023]. Available from: https://www.cdc.gov/ncezid/dhqp/index.html .
- 5.Berríos-Torres SI, Umscheid CA, Bratzler DW, Leas B, Stone EC, Kelz RR, Reinke CE, Morgan S, Solomkin JS, Mazuski JE, Dellinger EP, Itani KMF, Berbari EF, Segreti J, Parvizi J, Blanchard J, Allen G, Kluytmans JAJW, Donlan R, Schecter WP Healthcare Infection Control Practices Advisory Committee. Centers for Disease Control and Prevention Guideline for the Prevention of Surgical Site Infection, 2017. JAMA Surg. 2017;152:784–791. doi: 10.1001/jamasurg.2017.0904. [DOI] [PubMed] [Google Scholar]
- 6.Culver DH, Horan TC, Gaynes RP, Martone WJ, Jarvis WR, Emori TG, Banerjee SN, Edwards JR, Tolson JS, Henderson TS. Surgical wound infection rates by wound class, operative procedure, and patient risk index. National Nosocomial Infections Surveillance System. Am J Med. 1991;91:152S–157S. doi: 10.1016/0002-9343(91)90361-z. [DOI] [PubMed] [Google Scholar]
- 7.Neumayer L, Hosokawa P, Itani K, El-Tamer M, Henderson WG, Khuri SF. Multivariable predictors of postoperative surgical site infection after general and vascular surgery: results from the patient safety in surgery study. J Am Coll Surg. 2007;204:1178–1187. doi: 10.1016/j.jamcollsurg.2007.03.022. [DOI] [PubMed] [Google Scholar]
- 8.Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008;36:309–332. doi: 10.1016/j.ajic.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 9.Seidelman JL, Mantyh CR, Anderson DJ. Surgical Site Infection Prevention: A Review. JAMA. 2023;329:244–252. doi: 10.1001/jama.2022.24075. [DOI] [PubMed] [Google Scholar]
- 10.Alkaaki A, Al-Radi OO, Khoja A, Alnawawi A, Maghrabi A, Altaf A, Aljiffry M. Surgical site infection following abdominal surgery: a prospective cohort study. Can J Surg. 2019;62:111–117. doi: 10.1503/cjs.004818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gillespie BM, Harbeck E, Rattray M, Liang R, Walker R, Latimer S, Thalib L, Andersson AE, Griffin B, Ware R, Chaboyer W. Worldwide incidence of surgical site infections in general surgical patients: A systematic review and meta-analysis of 488,594 patients. Int J Surg. 2021;95:106136. doi: 10.1016/j.ijsu.2021.106136. [DOI] [PubMed] [Google Scholar]
- 12.GlobalSurg Collaborative. Laparoscopy in management of appendicitis in high-, middle-, and low-income countries: a multicenter, prospective, cohort study. Surg Endosc. 2018;32:3450–3466. doi: 10.1007/s00464-018-6064-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mengistu DA, Alemu A, Abdukadir AA, Mohammed Husen A, Ahmed F, Mohammed B, Musa I. Global Incidence of Surgical Site Infection Among Patients: Systematic Review and Meta-Analysis. Inquiry. 2023;60:469580231162549. doi: 10.1177/00469580231162549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Young H, Reese SM, Knepper B, Price CS. Impact of surveillance technique on reported rates of surgical site infection. Infect Control Hosp Epidemiol. 2015;36:594–596. doi: 10.1017/ice.2015.21. [DOI] [PubMed] [Google Scholar]
- 15.Caroff DA, Wang R, Zhang Z, Wolf R, Septimus E, Harris AD, Jackson SS, Poland RE, Hickok J, Huang SS, Platt R. The Limited Utility of Ranking Hospitals Based on Their Colon Surgery Infection Rates. Clin Infect Dis. 2021;72:90–98. doi: 10.1093/cid/ciaa012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Edwards JR, Peterson KD, Mu Y, Banerjee S, Allen-Bridson K, Morrell G, Dudeck MA, Pollock DA, Horan TC. National Healthcare Safety Network (NHSN) report: data summary for 2006 through 2008, issued December 2009. Am J Infect Control. 2009;37:783–805. doi: 10.1016/j.ajic.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention. 2019 National and State Healthcare-Associated Infections Progress Report. [cited 20 April 2023]. Available from: https://www.cdc.gov/hai/data/archive/2019-HAI-progress-report.html .
- 18.Foster D, Kethman W, Cai LZ, Weiser TG, Forrester JD. Surgical Site Infections after Appendectomy Performed in Low and Middle Human Development-Index Countries: A Systematic Review. Surg Infect (Larchmt) 2018;19:237–244. doi: 10.1089/sur.2017.188. [DOI] [PubMed] [Google Scholar]
- 19.ECDC Healthcare-associated infections: surgical site infections - Annual Epidemiological Report for 2018–2020. [cited 20 April 2023]. Available from: https://www.ecdc.europa.eu/en/publications-data/healthcare-associated-infections-surgical-site-annual-2018-2020 .
- 20.World Health Organization. Global guidelines on the prevention of surgical site infection. [cited 30 April 2022]. Available from: https://apps.who.int/iris/handle/10665/250680 .
- 21.Weiner-Lastinger LM, Abner S, Edwards JR, Kallen AJ, Karlsson M, Magill SS, Pollock D, See I, Soe MM, Walters MS, Dudeck MA. Antimicrobial-resistant pathogens associated with adult healthcare-associated infections: Summary of data reported to the National Healthcare Safety Network, 2015-2017. Infect Control Hosp Epidemiol. 2020;41:1–18. doi: 10.1017/ice.2019.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ling ML, Apisarnthanarak A, Madriaga G. The Burden of Healthcare-Associated Infections in Southeast Asia: A Systematic Literature Review and Meta-analysis. Clin Infect Dis. 2015;60:1690–1699. doi: 10.1093/cid/civ095. [DOI] [PubMed] [Google Scholar]
- 23.Nouetchognou JS, Ateudjieu J, Jemea B, Mesumbe EN, Mbanya D. Surveillance of nosocomial infections in the Yaounde University Teaching Hospital, Cameroon. BMC Res Notes. 2016;9:505. doi: 10.1186/s13104-016-2310-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gomaa K, Abdelraheim AR, El Gelany S, Khalifa EM, Yousef AM, Hassan H. Incidence, risk factors and management of post cesarean section surgical site infection (SSI) in a tertiary hospital in Egypt: a five year retrospective study. BMC Pregnancy Childbirth. 2021;21:634. doi: 10.1186/s12884-021-04054-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Raouf M, Ghazal T, Kassem M, Agamya A, Amer A. Surveillance of surgical-site infections and antimicrobial resistance patterns in a tertiary hospital in Alexandria, Egypt. J Infect Dev Ctries. 2020;14:277–283. doi: 10.3855/jidc.12124. [DOI] [PubMed] [Google Scholar]
- 26.Legesse Laloto T, Hiko Gemeda D, Abdella SH. Incidence and predictors of surgical site infection in Ethiopia: prospective cohort. BMC Infect Dis. 2017;17:119. doi: 10.1186/s12879-016-2167-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Misha G, Chelkeba L, Melaku T. Incidence, risk factors and outcomes of surgical site infections among patients admitted to Jimma Medical Center, South West Ethiopia: Prospective cohort study. Ann Med Surg (Lond) 2021;65:102247. doi: 10.1016/j.amsu.2021.102247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bediako-Bowan A, Owusu E, Debrah S, Kjerulf A, Newman MJ, Kurtzhals JAL, Mølbak K. Surveillance of surgical site infection in a teaching hospital in Ghana: a prospective cohort study. J Hosp Infect. 2020;104:321–327. doi: 10.1016/j.jhin.2020.01.004. [DOI] [PubMed] [Google Scholar]
- 29.Sway A, Wanyoro A, Nthumba P, Aiken A, Ching P, Maruta A, Gunturu R, Solomkin J. Prospective Cohort Study on Timing of Antimicrobial Prophylaxis for Post-Cesarean Surgical Site Infections. Surg Infect (Larchmt) 2020;21:552–557. doi: 10.1089/sur.2018.226. [DOI] [PubMed] [Google Scholar]
- 30.Flouchi R, El Far M, Hibatallah A, Elmniai A, Rhbibou I, Touzani I, El Hachlafi N, Fikri-Benbrahim K. Incidence of surgical site infections and prediction of risk factors in a hospital center in Morocco. J Infect Dev Ctries. 2022;16:1191–1198. doi: 10.3855/jidc.15289. [DOI] [PubMed] [Google Scholar]
- 31.Velin L, Umutesi G, Riviello R, Muwanguzi M, Bebell LM, Yankurije M, Faktor K, Nkurunziza T, Rukundo G, de Dieu Gatete J, Emil I, Hedt-Gauthier BL, Kateera F. Surgical Site Infections and Antimicrobial Resistance After Cesarean Section Delivery in Rural Rwanda. Ann Glob Health. 2021;87:77. doi: 10.5334/aogh.3413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carshon-Marsh R, Squire JS, Kamara KN, Sargsyan A, Delamou A, Camara BS, Manzi M, Guth JA, Khogali MA, Reid A, Kenneh S. Incidence of Surgical Site Infection and Use of Antibiotics among Patients Who Underwent Caesarean Section and Herniorrhaphy at a Regional Referral Hospital, Sierra Leone. Int J Environ Res Public Health. 2022;19 doi: 10.3390/ijerph19074048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lakoh S, Yi L, Sevalie S, Guo X, Adekanmbi O, Smalle IO, Williams N, Barrie U, Koroma C, Zhao Y, Kamara MN, Cummings-John C, Jiba DF, Namanaga ES, Deen B, Zhang J, Maruta A, Kallon C, Liu P, Wurie HR, Kanu JS, Deen GF, Samai M, Sahr F, Firima E. Incidence and risk factors of surgical site infections and related antibiotic resistance in Freetown, Sierra Leone: a prospective cohort study. Antimicrob Resist Infect Control. 2022;11:39. doi: 10.1186/s13756-022-01078-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Swart O, Esterhuizen TM, Voss M. The role of treatment delays in surgical site infection after appendicectomy in a South African rural regional hospital. S Afr Med J. 2021;111:271–275. doi: 10.7196/SAMJ.2021.v111i3.15231. [DOI] [PubMed] [Google Scholar]
- 35.Mawalla B, Mshana SE, Chalya PL, Imirzalioglu C, Mahalu W. Predictors of surgical site infections among patients undergoing major surgery at Bugando Medical Centre in Northwestern Tanzania. BMC Surg. 2011;11:21. doi: 10.1186/1471-2482-11-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ernest EC, Hellar A, Varallo J, Tibyehabwa L, Bertram MM, Fitzgerald L, Katoto A, Mshana S, Simba D, Gwitaba K, Boddu R, Alidina S, Giiti G, Kihunrwa A, Balandya B, Urassa D, Hussein Y, Damien C, Wackenreuter B, Barash D, Morrison M, Reynolds C, Christensen A, Makuwani A. Reducing surgical site infections and mortality among obstetric surgical patients in Tanzania: a pre-evaluation and postevaluation of a multicomponent safe surgery intervention. BMJ Glob Health. 2021;6 doi: 10.1136/bmjgh-2021-006788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Merzougui L, Marwen N, Hannachi H, Asma M, Ben Elhaj O, Waddah M, Fatnassi R. [Incidence and risk factors of surgical site infection following caesarean section in a Tunisian maternity unit] Sante Publique. 2018;30:339–347. doi: 10.3917/spub.183.0339. [DOI] [PubMed] [Google Scholar]
- 38.Ghali H, Ben Rejeb M, Ben Fredj S, Khefacha S, Latiri HA. Incidence and risk factors of surgical site infection in general surgery department of a Tunisian tertiary teaching hospital: a prospective observational study. Canadian J Infect Control. 2019;33:25–32. [Google Scholar]
- 39.Carvalho RLR, Campos CC, Franco LMC, Rocha AM, Ercole FF. Incidence and risk factors for surgical site infection in general surgeries. Rev Lat Am Enfermagem. 2017;25:e2848. doi: 10.1590/1518-8345.1502.2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ferraz ÁAB, Vasconcelos CFM, Santa-Cruz F, Aquino MAR, Buenos-Aires VG, Siqueira LT. Surgical site infection in bariatric surgery: results of a care bundle. Rev Col Bras Cir. 2019;46:e2252. doi: 10.1590/0100-6991e-20192252. [DOI] [PubMed] [Google Scholar]
- 41.Rouse T, Nascu P, Dawson C, Morris E. Incidence of surgical site infections after caesarean sections in a community hospital. Canadian J Infect Control. 2019:30–34. [Google Scholar]
- 42.Cram P, Cohen ME, Ko C, Landon BE, Hall B, Jackson TD. Surgical Outcomes in Canada and the United States: An Analysis of the ACS-NSQIP Clinical Registry. World J Surg. 2022;46:1039–1050. doi: 10.1007/s00268-022-06444-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Álvarez-Moreno C, Pérez-Fernández AM, Rosenthal VD, Quintero J, Chapeta-Parada E, Linares C, Pinilla-Martínez IF, Martínez-Saleg PA, Sierra P, Mindiola-Rochel AE. Surgical site infection rates in 4 cities in Colombia: findings of the International Nosocomial Infection Control Consortium (INICC) Am J Infect Control. 2014;42:1089–1092. doi: 10.1016/j.ajic.2014.06.010. [DOI] [PubMed] [Google Scholar]
- 44.Instituto Nacional de Salud | Colombia. Report on antimicrobial resistance in infections associated with medical-surgical procedures (ISO) Colombia, 2022. [cited 28 September 2023]. Available from: http://www.ins.gov.co/BibliotecaDigital/informe-de-resistencia-antimicrobiana-en-infecciones-asociadas-a-procedimientos-medico-quirurgicos-iso-colombia-2022.pdf . [Google Scholar]
- 45.Guanche Garcell H, González Valdés A, Pisonero Socias JJ, Gutiérrez García F, Pérez Díaz C. Incidencia de infección del sitio quirúrgico y cumplimiento de prácticas de prevención en apendicectomía y cirugía herniaria. Rev Cubana Cir. 2018;57 [Google Scholar]
- 46.Romero Viamonte K, Salvent Tames A, Sepúlveda Correa R, Rojo Manteca MV, Martín-Suárez A. Compliance with antibiotic prophylaxis guidelines in caesarean delivery: a retrospective, drug utilization study (indication-prescription type) at an Ecuadorian hospital. Antimicrob Resist Infect Control. 2021;10:12. doi: 10.1186/s13756-020-00843-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.José-Borjas E, Bejarano S, Martinez-Miranda PA, Toledo J, Campos G, Fajardo Lídice V, Lara E, Mendoza C, Alas Pineda CU. Presencia de infección de sitio quirúrgico en procedimientos gineco-obstétricos en un hospital de II nivel de atención, Honduras 2017-2018. Rev chil obstet ginecol. 2021;86:42–51. [Google Scholar]
- 48.Guzmán-García C, Flores-Barrientos OI, Juárez-Rojop IE, Robledo-Pascual JC, Baños-González MA, Tovilla-Záratee CA, Hernández-Díaz Y, González-Castro TB. Abdominal Surgical Site Infection Incidence and Risk Factors in a Mexican Population. Adv Skin Wound Care. 2019;32:1–6. doi: 10.1097/01.ASW.0000557833.80431.00. [DOI] [PubMed] [Google Scholar]
- 49.Hernández Centeno JR, Rivera Magaña G, Ramírez Barba ÉJ, Ávila Baylón R, Insensé Arana M. Colecistectomía subtotal como opción de manejo para colecistectomía difícil. Cir Gen. 2021;43 [Google Scholar]
- 50.Ramírez-Wong FM, Atencio-Espinoza T, Rosenthal VD, Ramirez E, Torres-Zegarra SL, Díaz Tavera ZR, Sarmiento López F, Silva Astete N, Campos Guevara F, Bazan Mendoza C, Valencia Ramírez A, Soto Pastrana J. Surgical Site Infections Rates in More Than 13,000 Surgical Procedures in Three Cities in Peru: Findings of the International Nosocomial Infection Control Consortium. Surg Infect (Larchmt) 2015;16:572–576. doi: 10.1089/sur.2014.201. [DOI] [PubMed] [Google Scholar]
- 51.Yerba K, Failoc-Rojas V, Zeña-Ñañez S, Valladares-Garrido M. Factors Associated with Surgical Site Infection in Post-Cesarean Section: A Case-Control Study in a Peruvian Hospital. Ethiop J Health Sci. 2020;30:95–100. doi: 10.4314/ejhs.v30i1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Centro Nacional de Epidemiología. Prevención y Control de Enfermedades - Indicadores epidemiológicos de referencia de las infecciones asociadasa la atención en salud, Perú 2019-2021. [cited 18 May 2023]. Available from: https://www.dge.gob.pe/portalnuevo/wp-content/uploads/2021/09/indicadores-IAAS_2019-2021.pdf .
- 53.Ministerio de Salud Pública. Sistema Nacional de vigilancia de las infecciones hospitalarias. [cited 30 April 2023]. Available from: https://www.gub.uy/ministerio-salud-publica/comunicacion/publicaciones/sistema-nacional-de-vigilancia-de-las-infecciones-hospitalarias .
- 54.Ministerio de Salud Publica. Vigilancia de infecciones asociadas a la asistencia en salud 2017-2021. [cited 30 September 2022]. Available from: https://www.gub.uy/ministerio-salud-publica/comunicacion/publicaciones/vigilancia-infecciones-asociadas-asistencia-salud-informacion-registrada .
- 55.Piñango S, Level L, Inchausti C. Incidencia de infección del sitio quirúrgico en el Servicio de Cirugía I, hospital Dr. Miguel Pérez Carreño. 2019-2021. Estudio observacional. Revista Venezolana de Cirugía. 2021;74 [Google Scholar]
- 56.Mu Y, Edwards JR, Horan TC, Berrios-Torres SI, Fridkin SK. Improving risk-adjusted measures of surgical site infection for the national healthcare safety network. Infect Control Hosp Epidemiol. 2011;32:970–986. doi: 10.1086/662016. [DOI] [PubMed] [Google Scholar]
- 57.Ely S, Rothenberg KA, Beattie G, Gologorsky RC, Huyser MR, Chang CK. Modern Elective Laparoscopic Cholecystectomy Carries Extremely Low Postoperative Infection Risk. J Surg Res. 2020;246:506–511. doi: 10.1016/j.jss.2019.09.038. [DOI] [PubMed] [Google Scholar]
- 58.Zhang XF, Chen J, Wang PG, Luo SM, Liu NX, Li XM, He XL, Wang Y, Bi XG, Zhang P, Lv ZC, Zhou B, Mai W, Wu H, Hu Y, Wang DR, Luo FW, Xia LG, Lai JJ, Zhang DM, Wang Q, Han G, Wu XW, Ren JA. [Surgical site infection after abdominal surgery in China: a multicenter cross-sectional study] Zhonghua Wei Chang Wai Ke Za Zhi. 2020;23:1036–1042. doi: 10.3760/cma.j.cn.441530-20200810-00470. [DOI] [PubMed] [Google Scholar]
- 59.Zhang X, Wang Z, Chen J, Wang P, Luo S, Xu X, Mai W, Li G, Wang G, Wu X, Ren J. Incidence and risk factors of surgical site infection following colorectal surgery in China: a national cross-sectional study. BMC Infect Dis. 2020;20:837. doi: 10.1186/s12879-020-05567-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang Z, Chen J, Wang P, Jie Z, Jin W, Wang G, Li J, Ren J. Surgical Site Infection After Gastrointestinal Surgery in China: A Multicenter Prospective Study. J Surg Res. 2019;240:206–218. doi: 10.1016/j.jss.2019.03.017. [DOI] [PubMed] [Google Scholar]
- 61.Li L, Cui H. The risk factors and care measures of surgical site infection after cesarean section in China: a retrospective analysis. BMC Surg. 2021;21:248. doi: 10.1186/s12893-021-01154-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dsouza R, Varghese G, Mittal R, Jesudason MR. Perineal Wound Outcomes after Extralevator Abdominoperineal Excision for Rectal Adenocarcinoma- A Tertiary Care Centre Experience: Type of article: Original full length article. Wound Med. 2020;29:100184. [Google Scholar]
- 63.Gupta S, Manchanda V, Sachdev P, Kumar Saini R, Joy M. Study of incidence and risk factors of surgical site infections in lower segment caesarean section cases of tertiary care hospital of north India. Indian J Med Microbiol. 2021;39:1–5. doi: 10.1016/j.ijmmb.2020.11.005. [DOI] [PubMed] [Google Scholar]
- 64.Singh S, Chakravarthy M, Rosenthal VD, Myatra SN, Dwivedy A, Bagasrawala I, Munshi N, Shah S, Panigrahi B, Sood S, Kumar-Nair P, Radhakrishnan K, Gokul BN, Sukanya R, Pushparaj L, Pramesh CS, Shrikhande SV, Gulia A, Puri A, Moiyadi A, Divatia JV, Kelkar R, Biswas S, Raut S, Sampat S, Shetty S, Binu S, Pinto P, Arora S, Kamble A, Kumari N, Mendonca A, Singhal T, Naik R, Kothari V, Sharma B, Verma N, Khanna DK, Chacko F. Surgical site infection rates in six cities of India: findings of the International Nosocomial Infection Control Consortium (INICC) Int Health. 2015;7:354–359. doi: 10.1093/inthealth/ihu089. [DOI] [PubMed] [Google Scholar]
- 65.Masoudifar M, Gouya MM, Pezeshki Z, Eshrati B, Afhami S, Farzami MR, Seifi A. Health care-associated infections, including device-associated infections, and antimicrobial resistance in Iran: The national update for 2018. J Prev Med Hyg. 2021;62:E943–E949. doi: 10.15167/2421-4248/jpmh2021.62.4.1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nasiri N, Sharifi A, Ghasemzadeh I, Khalili M, Karamoozian A, Khalooei A, Beigzadeh A, Haghdoost A, Sharifi H. Incidence, accuracy, and barriers of diagnosing healthcare-associated infections: a case study in southeast Iran. BMC Infect Dis. 2023;23:171. doi: 10.1186/s12879-023-08122-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Morikane K, Konishi T, Harihara Y, Nishioka M, Kobayashi H. Implementation and establishment of nationwide surgical site infections surveillance in Japan. Am J Infect Control. 2005;33:E175–E176. [Google Scholar]
- 68.Morikane K, Honda H, Yamagishi T, Suzuki S, Aminaka M. Factors associated with surgical site infection in colorectal surgery: the Japan nosocomial infections surveillance. Infect Control Hosp Epidemiol. 2014;35:660–666. doi: 10.1086/676438. [DOI] [PubMed] [Google Scholar]
- 69.Kajihara T, Yahara K, Hirabayashi A, Hosaka Y, Kitamura N, Sugai M, Shibayama K. Association between the proportion of laparoscopic approaches for digestive surgeries and the incidence of consequent surgical site infections, 2009-2019: A retrospective observational study based on national surveillance data in Japan. PLoS One. 2023;18:e0281838. doi: 10.1371/journal.pone.0281838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hamza WS, Salama MF, Morsi SS, Abdo NM, Al-Fadhli MA. Benchmarking for surgical site infections among gastrointestinal surgeries and related risk factors: multicenter study in Kuwait. Infect Drug Resist. 2018;11:1373–1381. doi: 10.2147/IDR.S167213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Regmi A, Ojha N, Singh M, Ghimire A, Kharel N. Risk Factors Associated with Surgical Site Infection following Cesarean Section in Tertiary Care Hospital, Nepal. Int J Reprod Med. 2022;2022:4442453. doi: 10.1155/2022/4442453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jamal A, Shakeel O, Mohsin J, Malik AA, Haq IU, Begum S, Khan SM, Hanif F, Kabir SI, Syed AA. Pancreaticoduodenectomy: Outcomes of a complex surgical procedure from a developing country. Pancreatology. 2020;20:1534–1539. doi: 10.1016/j.pan.2020.08.013. [DOI] [PubMed] [Google Scholar]
- 73.Khan FU, Fang Y, Khan Z, Khan FU, Malik ZI, Ahmed N, Khan AH, Rehman A. Occurrence, associated risk factors, and treatment of surgical site infections in Pakistan. Eur J Inflamm. 2020:18. [Google Scholar]
- 74.Henarejos V, O'Connor K, Barrasa A, Villalonga A, Pastor C, Puyana JC, Merck B. Implementing a mHealth-Based Patient and Nurse Educational Program to Reduce Wound Infection in Rural Philippines. Ann Glob Health. 2022;88:76. doi: 10.5334/aogh.3834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Choi HJ, Adiyani L, Sung J, Choi JY, Kim HB, Kim YK, Kwak YG, Yoo H, Lee SO, Han SH, Kim SR, Kim TH, Lee HM, Chun HK, Kim JS, Yoo JD, Koo HS, Cho EH, Lee KW Korean Nosocomial Infections Surveillance System (KONIS) Five-year decreased incidence of surgical site infections following gastrectomy and prosthetic joint replacement surgery through active surveillance by the Korean Nosocomial Infection Surveillance System. J Hosp Infect. 2016;93:339–346. doi: 10.1016/j.jhin.2015.12.021. [DOI] [PubMed] [Google Scholar]
- 76.Taiwan CDC. Taiwan Healthcare-associated infection and Antimicrobial resistance Surveillance System. [cited 30 April 2023]. Available from: https://www.cdc.gov.tw/En/Category/Page/J63NmsvevBg2u3I2qYBenw .
- 77.Chungsiriwattana W, Sangkhathat S, Kongkamol C, Suwalak N, Phainuphong P, Komet O, Darayon R. Decreasing Trend of Surgical Site Infections among Surgical Patients in a University Hospital in Thailand after an Active Surveillance Program. Surg Infect (Larchmt) 2019;20:382–389. doi: 10.1089/sur.2018.124. [DOI] [PubMed] [Google Scholar]
- 78.Leblebicioglu H, Erben N, Rosenthal VD, Sener A, Uzun C, Senol G, Ersoz G, Demirdal T, Duygu F, Willke A, Sirmatel F, Oztoprak N, Koksal I, Oncul O, Gurbuz Y, Güçlü E, Turgut H, Yalcin AN, Ozdemir D, Kendirli T, Aslan T, Esen S, Ulger F, Dilek A, Yilmaz H, Sunbul M, Ozgunes I, Usluer G, Otkun M, Kaya A, Kuyucu N, Kaya Z, Meric M, Azak E, Yýlmaz G, Kaya S, Ulusoy H, Haznedaroglu T, Gorenek L, Acar A, Tutuncu E, Karabay O, Kaya G, Sacar S, Sungurtekin H, Uğurcan D, Turhan O, Gumus E, Dursun O, Geyik MF, Şahin A, Erdogan S, Ince E, Karbuz A, Çiftçi E, Taşyapar N, Güneş M. Surgical site infection rates in 16 cities in Turkey: findings of the International Nosocomial Infection Control Consortium (INICC) Am J Infect Control. 2015;43:48–52. doi: 10.1016/j.ajic.2014.09.017. [DOI] [PubMed] [Google Scholar]
- 79.Alnajjar MS, Alashker DA. Surgical site infections following caesarean sections at Emirati teaching hospital: Incidence and implicated factors. Sci Rep. 2020;10:18702. doi: 10.1038/s41598-020-75582-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.UK Health Security Agency. Surveillance of surgical site infections in NHS hospitals in England: April 2021 to March 2022. [cited 30 April 2023]. Available from: https://1821938-1-SSI-annual-report-2020-to-2021.pdf .
- 81.López Barrachina R, de la Cruz Tabares E, Guzmán Collado IT. Incidence of surgical site infection in colon surgery according to RENAVE methodology: Prospective study 2017-2019. Cir Esp (Engl Ed) 2021;99:34–40. doi: 10.1016/j.ciresp.2020.05.008. [DOI] [PubMed] [Google Scholar]
- 82.Colás-Ruiz E, Del-Moral-Luque JA, Gil-Yonte P, Fernández-Cebrián JM, Alonso-García M, Villar-Del-Campo MC, Durán-Poveda M, Rodríguez-Caravaca G. Incidence of surgical site infection and risk factors in rectal surgery: A prospective cohort study. Cir Esp (Engl Ed) 2018;96:640–647. doi: 10.1016/j.ciresp.2018.06.007. [DOI] [PubMed] [Google Scholar]
- 83.Rodríguez-Caravaca G, Gil-Yonte P, Del-Moral-Luque JA, Lucas WC, Fernández-Cebrián JM, Durán-Poveda M. Rates of Surgical Site Infection in Cholecystectomy: Comparison between a University Teaching Hospital, Madrid Region, Spain, and USA Rates. Rev Invest Clin. 2017;69:336–343. doi: 10.24875/RIC.17002197. [DOI] [PubMed] [Google Scholar]
- 84.Sommerstein R, Vuichard D, Metsini A, Eisenring MC, Zingg W, Marschall J, Schlegel M, Troillet N, Andreas Widmer for Swissnoso. First report of Swissnoso on the epidemiology of healthcare-associated infections in Switzerland since 2017. [cited 30 April 2023]. Available from: https://www.swissnoso.ch/fileadmin/swissnoso/Dokumente/5_Forschung_und_Entwicklung/8_Swissnoso_Publikationen/Swissnoso_report_Epidemiological_HAI_situation_in_Switzerland_since_2017_per_Aug_2020.pdf .
- 85.Worth LJ, Bull AL, Spelman T, Brett J, Richards MJ. Diminishing surgical site infections in Australia: time trends in infection rates, pathogens and antimicrobial resistance using a comprehensive Victorian surveillance program, 2002-2013. Infect Control Hosp Epidemiol. 2015;36:409–416. doi: 10.1017/ice.2014.70. [DOI] [PubMed] [Google Scholar]
- 86.Sekirov I, Russell SL, Antunes LC, Finlay BB. Gut microbiota in health and disease. Physiol Rev. 2010;90:859–904. doi: 10.1152/physrev.00045.2009. [DOI] [PubMed] [Google Scholar]
- 87.Jandhyala SM, Talukdar R, Subramanyam C, Vuyyuru H, Sasikala M, Nageshwar Reddy D. Role of the normal gut microbiota. World J Gastroenterol. 2015;21:8787–8803. doi: 10.3748/wjg.v21.i29.8787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kim SI. Bacterial infection after liver transplantation. World J Gastroenterol. 2014;20:6211–6220. doi: 10.3748/wjg.v20.i20.6211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fishman JA. Infections in immunocompromised hosts and organ transplant recipients: essentials. Liver Transpl. 2011;17 Suppl 3:S34–S37. doi: 10.1002/lt.22378. [DOI] [PubMed] [Google Scholar]
- 90.Russell DL, Flood A, Zaroda TE, Acosta C, Riley MM, Busuttil RW, Pegues DA. Outcomes of colonization with MRSA and VRE among liver transplant candidates and recipients. Am J Transplant. 2008;8:1737–1743. doi: 10.1111/j.1600-6143.2008.02304.x. [DOI] [PubMed] [Google Scholar]
- 91.Herati RS, Blumberg EA. Losing ground: multidrug-resistant bacteria in solid-organ transplantation. Curr Opin Infect Dis. 2012;25:445–449. doi: 10.1097/QCO.0b013e328354f192. [DOI] [PubMed] [Google Scholar]
- 92.Livermore DM. Current epidemiology and growing resistance of gram-negative pathogens. Korean J Intern Med. 2012;27:128–142. doi: 10.3904/kjim.2012.27.2.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Esposito S, Bassetti M, Borre' S, Bouza E, Dryden M, Fantoni M, Gould IM, Leoncini F, Leone S, Milkovich G, Nathwani D, Segreti J, Sganga G, Unal S, Venditti M Italian Society of Infectious Tropical Diseases; International Society of Chemotherapy. Diagnosis and management of skin and soft-tissue infections (SSTI): a literature review and consensus statement on behalf of the Italian Society of Infectious Diseases and International Society of Chemotherapy. J Chemother. 2011;23:251–262. doi: 10.1179/joc.2011.23.5.251. [DOI] [PubMed] [Google Scholar]
- 94.Esposito S, Leone S, Carosi G. Analysis of current guidelines for intra-abdominal infections. J Chemother. 2009;21 Suppl 1:30–35. doi: 10.1179/joc.2009.21.Supplement-1.30. [DOI] [PubMed] [Google Scholar]
- 95.Edmiston CE Jr, McBain AJ, Roberts C, Leaper D. Clinical and microbiological aspects of biofilm-associated surgical site infections. Adv Exp Med Biol. 2015;830:47–67. doi: 10.1007/978-3-319-11038-7_3. [DOI] [PubMed] [Google Scholar]
- 96.Gurusamy KS, Toon CD, Allen VB, Davidson BR. Continuous versus interrupted skin sutures for non-obstetric surgery. Cochrane Database Syst Rev. 2014:CD010365. doi: 10.1002/14651858.CD010365.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Konstantelias AA, Andriakopoulou CS, Mourgela S. Triclosan-coated sutures for the prevention of surgical-site infections: a meta-analysis. Acta Chir Belg. 2017;117:137–148. doi: 10.1080/00015458.2017.1287396. [DOI] [PubMed] [Google Scholar]
- 98.Fonnes S, Holzknecht BJ, Arpi M, Rosenberg J. Characterisation and Safety of Intraperitoneal Perioperative Administration of Antibacterial Agents: A Systematic Review. Drug Res (Stuttg) 2017;67:688–697. doi: 10.1055/s-0043-109565. [DOI] [PubMed] [Google Scholar]
- 99.Mueller TC, Loos M, Haller B, Mihaljevic AL, Nitsche U, Wilhelm D, Friess H, Kleeff J, Bader FG. Intra-operative wound irrigation to reduce surgical site infections after abdominal surgery: a systematic review and meta-analysis. Langenbecks Arch Surg. 2015;400:167–181. doi: 10.1007/s00423-015-1279-x. [DOI] [PubMed] [Google Scholar]
- 100.De Simone B, Sartelli M, Coccolini F, Ball CG, Brambillasca P, Chiarugi M, Campanile FC, Nita G, Corbella D, Leppaniemi A, Boschini E, Moore EE, Biffl W, Peitzmann A, Kluger Y, Sugrue M, Fraga G, Di Saverio S, Weber D, Sakakushev B, Chiara O, Abu-Zidan FM, Ten Broek R, Kirkpatrick AW, Wani I, Coimbra R, Baiocchi GL, Kelly MD, Ansaloni L, Catena F. Correction to: Intraoperative surgical site infection control and prevention: a position paper and future addendum to WSES intra-abdominal infections guidelines. World J Emerg Surg. 2021;16:18. doi: 10.1186/s13017-021-00361-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kang SI, Oh HK, Kim MH, Kim MJ, Kim DW, Kim HJ, Kang SB. Systematic review and meta-analysis of randomized controlled trials of the clinical effectiveness of impervious plastic wound protectors in reducing surgical site infections in patients undergoing abdominal surgery. Surgery. 2018;164:939–945. doi: 10.1016/j.surg.2018.05.024. [DOI] [PubMed] [Google Scholar]
- 102.Arer IM, Yabanoglu H, Aytac HO, Ezer A. The effect of subcutaneous suction drains on surgical site infection in open abdominal surgery A prospective randomized study. Ann Ital Chir. 2016;87:49–55. [PubMed] [Google Scholar]
- 103.Partecke LI, Goerdt AM, Langner I, Jaeger B, Assadian O, Heidecke CD, Kramer A, Huebner NO. Incidence of microperforation for surgical gloves depends on duration of wear. Infect Control Hosp Epidemiol. 2009;30:409–414. doi: 10.1086/597062. [DOI] [PubMed] [Google Scholar]
- 104.Danno K, Matsuda C, Miyazaki S, Komori T, Nakanishi M, Motoori M, Kashiwazaki M, Fujitani K. Efficacy of Negative-Pressure Wound Therapy for Preventing Surgical Site Infections after Surgery for Peritonitis Attributable to Lower-Gastrointestinal Perforation: A Single-Institution Experience. Surg Infect (Larchmt) 2018;19:711–716. doi: 10.1089/sur.2018.134. [DOI] [PubMed] [Google Scholar]
- 105.Bhangu A, Singh P, Lundy J, Bowley DM. Systemic review and meta-analysis of randomized clinical trials comparing primary vs delayed primary skin closure in contaminated and dirty abdominal incisions. JAMA Surg. 2013;148:779–786. doi: 10.1001/jamasurg.2013.2336. [DOI] [PubMed] [Google Scholar]
- 106.Kurz A, Sessler DI, Lenhardt R. Perioperative normothermia to reduce the incidence of surgical-wound infection and shorten hospitalization. Study of Wound Infection and Temperature Group. N Engl J Med. 1996;334:1209–1215. doi: 10.1056/NEJM199605093341901. [DOI] [PubMed] [Google Scholar]
- 107.Cohen B, Schacham YN, Ruetzler K, Ahuja S, Yang D, Mascha EJ, Barclay AB, Hung MH, Sessler DI. Effect of intraoperative hyperoxia on the incidence of surgical site infections: a meta-analysis. Br J Anaesth. 2018;120:1176–1186. doi: 10.1016/j.bja.2018.02.027. [DOI] [PubMed] [Google Scholar]
- 108.Hecker A, Reichert M, Reuß CJ, Schmoch T, Riedel JG, Schneck E, Padberg W, Weigand MA, Hecker M. Intra-abdominal sepsis: new definitions and current clinical standards. Langenbecks Arch Surg. 2019;404:257–271. doi: 10.1007/s00423-019-01752-7. [DOI] [PubMed] [Google Scholar]
- 109.Ceresoli M, Lo Bianco G, Gianotti L, Nespoli L. Inflammation management in acute diverticulitis: current perspectives. J Inflamm Res. 2018;11:239–246. doi: 10.2147/JIR.S142990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Leone S, Damiani G, Pezone I, Kelly ME, Cascella M, Alfieri A, Pace MC, Fiore M. New antimicrobial options for the management of complicated intra-abdominal infections. Eur J Clin Microbiol Infect Dis. 2019;38:819–827. doi: 10.1007/s10096-019-03533-y. [DOI] [PubMed] [Google Scholar]
- 111.Leone S, Cascella M, Pezone I, Fiore M. New antibiotics for the treatment of serious infections in intensive care unit patients. Curr Med Res Opin. 2019;35:1331–1334. doi: 10.1080/03007995.2019.1583025. [DOI] [PubMed] [Google Scholar]
- 112.Fiore M, Taccone FS, Leone S. Choosing the appropriate pharmacotherapy for multidrug-resistant Gram positive infections. Expert Opin Pharmacother. 2018;19:1517–1521. doi: 10.1080/14656566.2018.1512584. [DOI] [PubMed] [Google Scholar]
- 113.Esposito S, Capuano A, Noviello S, Mazzeo F, Ianniello F, Filippelli A, Rossi F, Leone S. Modification of patients' endogenous bacterial flora during hospitalization in a large teaching hospital in Naples. J Chemother. 2003;15:568–573. doi: 10.1179/joc.2003.15.6.568. [DOI] [PubMed] [Google Scholar]
- 114.Noviello S, Ianniello F, Leone S, Fiore M, Esposito S. In vitro activity of tigecycline: MICs, MBCs, time-kill curves and post-antibiotic effect. J Chemother. 2008;20:577–580. doi: 10.1179/joc.2008.20.5.577. [DOI] [PubMed] [Google Scholar]
- 115.Leone S, Stefani S, Venditti M, Grossi P, Colizza S, De Gasperi A, Scaglione F, Sganga G, Esposito S Italian Intra-abdominal Infections Working Group. Intra-abdominal infections: model of antibiotic stewardship in an era with limited antimicrobial options. Int J Antimicrob Agents. 2011;38:271–272. doi: 10.1016/j.ijantimicag.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 116.Solomkin JS, Mazuski JE, Bradley JS, Rodvold KA, Goldstein EJ, Baron EJ, O'Neill PJ, Chow AW, Dellinger EP, Eachempati SR, Gorbach S, Hilfiker M, May AK, Nathens AB, Sawyer RG, Bartlett JG. Diagnosis and management of complicated intra-abdominal infection in adults and children: guidelines by the Surgical Infection Society and the Infectious Diseases Society of America. Clin Infect Dis. 2010;50:133–164. doi: 10.1086/649554. [DOI] [PubMed] [Google Scholar]
- 117.Fiore M, Gentile I, Maraolo AE, Leone S, Simeon V, Chiodini P, Pace MC, Gustot T, Taccone FS. Are third-generation cephalosporins still the empirical antibiotic treatment of community-acquired spontaneous bacterial peritonitis? A systematic review and meta-analysis. Eur J Gastroenterol Hepatol. 2018;30:329–336. doi: 10.1097/MEG.0000000000001057. [DOI] [PubMed] [Google Scholar]
- 118.Solomkin J, Hershberger E, Miller B, Popejoy M, Friedland I, Steenbergen J, Yoon M, Collins S, Yuan G, Barie PS, Eckmann C. Ceftolozane/Tazobactam Plus Metronidazole for Complicated Intra-abdominal Infections in an Era of Multidrug Resistance: Results From a Randomized, Double-Blind, Phase 3 Trial (ASPECT-cIAI) Clin Infect Dis. 2015;60:1462–1471. doi: 10.1093/cid/civ097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wunderink RG, Giamarellos-Bourboulis EJ, Rahav G, Mathers AJ, Bassetti M, Vazquez J, Cornely OA, Solomkin J, Bhowmick T, Bishara J, Daikos GL, Felton T, Furst MJL, Kwak EJ, Menichetti F, Oren I, Alexander EL, Griffith D, Lomovskaya O, Loutit J, Zhang S, Dudley MN, Kaye KS. Effect and Safety of Meropenem-Vaborbactam versus Best-Available Therapy in Patients with Carbapenem-Resistant Enterobacteriaceae Infections: The TANGO II Randomized Clinical Trial. Infect Dis Ther. 2018;7:439–455. doi: 10.1007/s40121-018-0214-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Motsch J, Murta de Oliveira C, Stus V, Köksal I, Lyulko O, Boucher HW, Kaye KS, File TM, Brown ML, Khan I, Du J, Joeng HK, Tipping RW, Aggrey A, Young K, Kartsonis NA, Butterton JR, Paschke A. RESTORE-IMI 1: A Multicenter, Randomized, Double-blind Trial Comparing Efficacy and Safety of Imipenem/Relebactam vs Colistin Plus Imipenem in Patients With Imipenem-nonsusceptible Bacterial Infections. Clin Infect Dis. 2020;70:1799–1808. doi: 10.1093/cid/ciz530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Solomkin J, Evans D, Slepavicius A, Lee P, Marsh A, Tsai L, Sutcliffe JA, Horn P. Assessing the Efficacy and Safety of Eravacycline vs Ertapenem in Complicated Intra-abdominal Infections in the Investigating Gram-Negative Infections Treated With Eravacycline (IGNITE 1) Trial: A Randomized Clinical Trial. JAMA Surg. 2017;152:224–232. doi: 10.1001/jamasurg.2016.4237. [DOI] [PubMed] [Google Scholar]
- 122.Solomkin JS, Gardovskis J, Lawrence K, Montravers P, Sway A, Evans D, Tsai L. IGNITE4: Results of a Phase 3, Randomized, Multicenter, Prospective Trial of Eravacycline vs Meropenem in the Treatment of Complicated Intraabdominal Infections. Clin Infect Dis. 2019;69:921–929. doi: 10.1093/cid/ciy1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Felice VG, Efimova E, Izmailyan S, Napolitano LM, Chopra T. Efficacy and Tolerability of Eravacycline in Bacteremic Patients with Complicated Intra-Abdominal Infection: A Pooled Analysis from the IGNITE1 and IGNITE4 Studies. Surg Infect (Larchmt) 2021;22:556–561. doi: 10.1089/sur.2020.241. [DOI] [PubMed] [Google Scholar]
- 124.Bassetti M, Echols R, Matsunaga Y, Ariyasu M, Doi Y, Ferrer R, Lodise TP, Naas T, Niki Y, Paterson DL, Portsmouth S, Torre-Cisneros J, Toyoizumi K, Wunderink RG, Nagata TD. Efficacy and safety of cefiderocol or best available therapy for the treatment of serious infections caused by carbapenem-resistant Gram-negative bacteria (CREDIBLE-CR): a randomised, open-label, multicentre, pathogen-focused, descriptive, phase 3 trial. Lancet Infect Dis. 2021;21:226–240. doi: 10.1016/S1473-3099(20)30796-9. [DOI] [PubMed] [Google Scholar]
- 125.McKinnell JA, Dwyer JP, Talbot GH, Connolly LE, Friedland I, Smith A, Jubb AM, Serio AW, Krause KM, Daikos GL CARE Study Group. Plazomicin for Infections Caused by Carbapenem-Resistant Enterobacteriaceae. N Engl J Med. 2019;380:791–793. doi: 10.1056/NEJMc1807634. [DOI] [PubMed] [Google Scholar]
- 126.Knitsch W, Vincent JL, Utzolino S, François B, Dinya T, Dimopoulos G, Özgüneş İ, Valía JC, Eggimann P, León C, Montravers P, Phillips S, Tweddle L, Karas A, Brown M, Cornely OA. A randomized, placebo-controlled trial of preemptive antifungal therapy for the prevention of invasive candidiasis following gastrointestinal surgery for intra-abdominal infections. Clin Infect Dis. 2015;61:1671–1678. doi: 10.1093/cid/civ707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.De Waele JJ, Vogelaers D, Blot S, Colardyn F. Fungal infections in patients with severe acute pancreatitis and the use of prophylactic therapy. Clin Infect Dis. 2003;37:208–213. doi: 10.1086/375603. [DOI] [PubMed] [Google Scholar]
- 128.Dutescu IA, Hillier SA. Encouraging the Development of New Antibiotics: Are Financial Incentives the Right Way Forward? A Systematic Review and Case Study. Infect Drug Resist. 2021;14:415–434. doi: 10.2147/IDR.S287792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Li P, Wang Y, Li H, Cheng B, Wu S, Ye H, Ma D, Fang X International Surgical Outcomes Study (ISOS) group in China. Prediction of postoperative infection in elderly using deep learning-based analysis: an observational cohort study. Aging Clin Exp Res. 2023;35:639–647. doi: 10.1007/s40520-022-02325-3. [DOI] [PMC free article] [PubMed] [Google Scholar]