Extended Data Fig 5. Evaluation of heterotypic driving force on the formation of DNA condensate and the partition of RNAP into the DNA condensate.
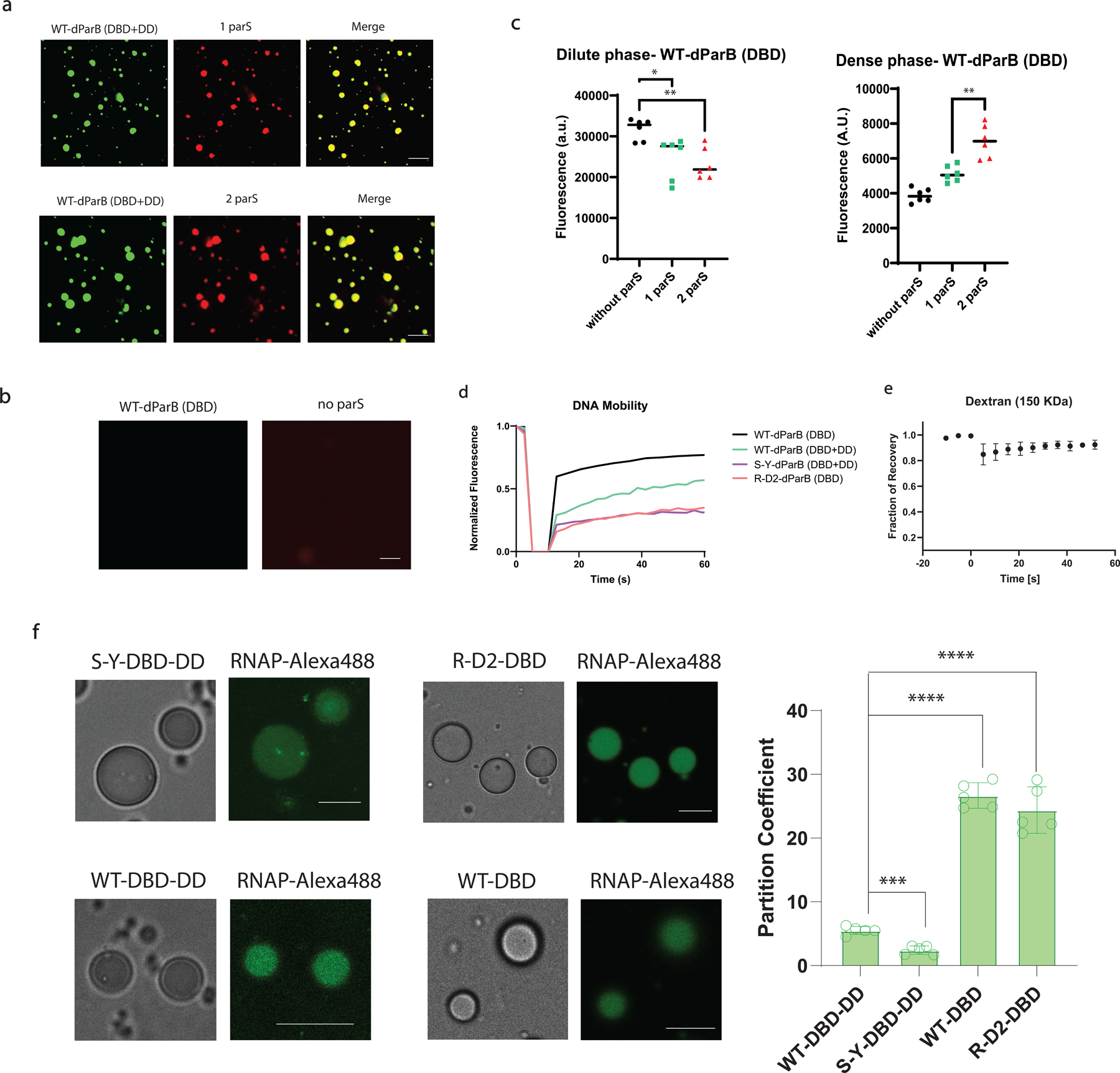
a, DNA condensate constituted by 500 nM of RLPWT-DBD-DD (doped with 10% RLPWT-DBD-DD -sfGFP) and 25 nM cy3-DNA containing one or two parS sites. Scale bar is 10 μm.
b, Mixture of 500 nM of RLPWT-DBD (doped with 10% RLPWT-DBD-sfGFP) and 25 nM cy3-DNA containing no parS site did not result the formation of condensate. Scale bar is 10 μm.
c, Fluorescence based sedimentation assay measurements of the dilute/dense phase concentrations of RLPWT-DBD (doped with 10% RLPWT-DBD-sfGFP) in the presence of 25 nM DNA with different number of parS binding sites. For measuring the dilute phase, the fluorescence was directly quantified from the supernatant after sedimentation (see Supplementary Methods). For measuring the dense phase, 1 µL of the dense phase was extracted and dissolved into 49 µL buffer before taking the measurements. n=6 independent experiments. *, p=0.0134; **, p=0.0012; **, p=0.0015 by a two-tailed unpair t-test. Error bar represents standard deviation.
d, Normalized fluorescence recovery curve of DNA channel based on the DNA condensate formed by different components. For evaluation of the mobility of cy3-DNA, the protein component was not doped with sfGFP labeled protein.
e, Normalized fluorescence recovery curve of Dextran (150 KDa) channel based on the DNA condensate formed in Fig 3g. n=3 independent experiments. Data point represents mean ± SD.
f, Evaluation of partition coefficient of Alexa488-labeled T7 RNAP into different DNA condensates containing 1 µM of RLP-dParB and 25 nM of DNA containing one parS site. n=5 independent experiments. Bar graph represents mean ± SD. ****, p<0.0001; ***, p<0.0005 by a two-tailed unpair t-test
