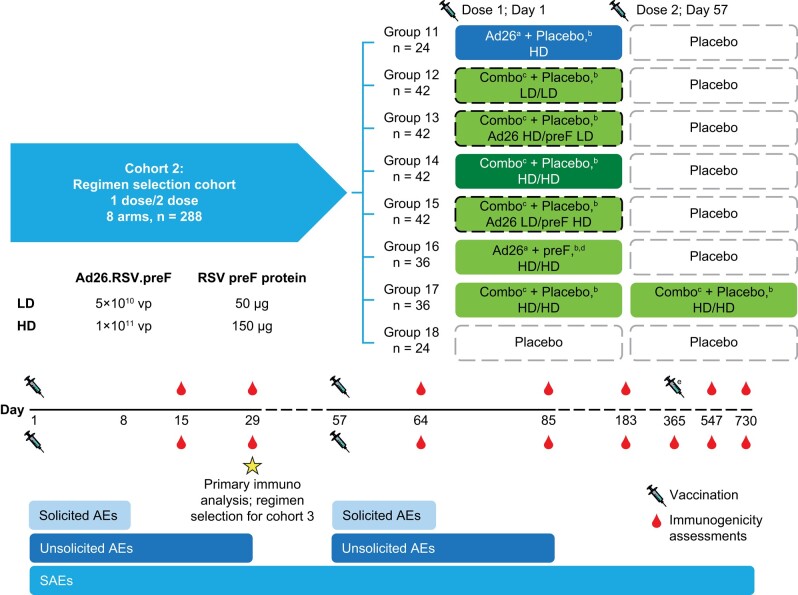
Figure 1.
Study schematic: cohort 2. Ad26, adenovector 26; AE, adverse event; HD, high dose; LD, low dose; preF, prefusion conformation-stabilized RSV F protein; RSV, respiratory syncytial virus; SAE, serious adverse event; vp, viral particle. aAd26.RSV.preF. bInjections given in opposite arms. cA combination injection of Ad26.RSV.preF and recombinant RSV preF protein at the specified dosages. dRecombinant RSV preF protein. eCohort 1 received an additional dose at day 365.