Abstract
OBJECTIVES:
The objective of this narrative review was to address common obstacles encountered in the ICU to acquiring quality and interpretable images using point-of-care echocardiography.
DATA SOURCES:
Detailed searches were performed using PubMed and Ovid Medline using medical subject headings and keywords on topics related to patient positioning, IV echo contrast, alternative subcostal views, right ventricular outflow tract (RVOT) hemodynamics, and point-of-care transesophageal echocardiography. Articles known to the authors were also selected based on expert opinion.
STUDY SELECTION:
Articles specific to patient positioning, IV echo contrast, alternative subcostal views, RVOT hemodynamics, and point-of-care transesophageal echocardiography were considered.
DATA EXTRACTION:
One author screened titles and extracted relevant data while two separate authors independently reviewed selected articles.
DATA SYNTHESIS:
Impediments to acquiring quality and interpretable images in critically ill patients are common. Notably, body habitus, intra-abdominal hypertension, dressings or drainage tubes, postoperative sternotomies, invasive mechanical ventilation, and the presence of subcutaneous emphysema or lung hyperinflation are commonly encountered obstacles in transthoracic image acquisition in the ICU. Despite these obstacles, the bedside clinician may use obstacle-specific maneuvers to enhance image acquisition. These may include altering patient positioning, respiratory cycle timing, expanding the subcostal window to include multilevel short-axis views for use in the assessment of RV systolic function and hemodynamics, coronal transhepatic view of the inferior vena cava, and finally point-of-care transesophageal echocardiography.
CONCLUSIONS:
Despite common obstacles to point-of-care echocardiography in critically ill patients, the beside sonographer may take an obstacle-specific stepwise approach to enhance image acquisition in difficult-to-image patients.
Keywords: critical care, hemodynamics, transesophageal echocardiography, transthoracic echocardiography, ultrasound
KEY POINTS
Question: Impediments to point-of-care echocardiographic imaging are commonly encountered in critically ill patients in the ICU. The aim of this narrative review was to provide a stepwise approach to how the bedside clinician may address these impediments.
Findings: Methods to enhance echocardiographic imaging in critically ill patients include optimizing patient positioning, using advanced methods to enhance subcostal window views, including for hemodynamic assessments, as well as the use of point-of-care transesophageal echocardiography.
Meaning: Advanced echocardiographic maneuvers can be used in the ICU to improve diagnostic evaluation with point-of-care echocardiography.
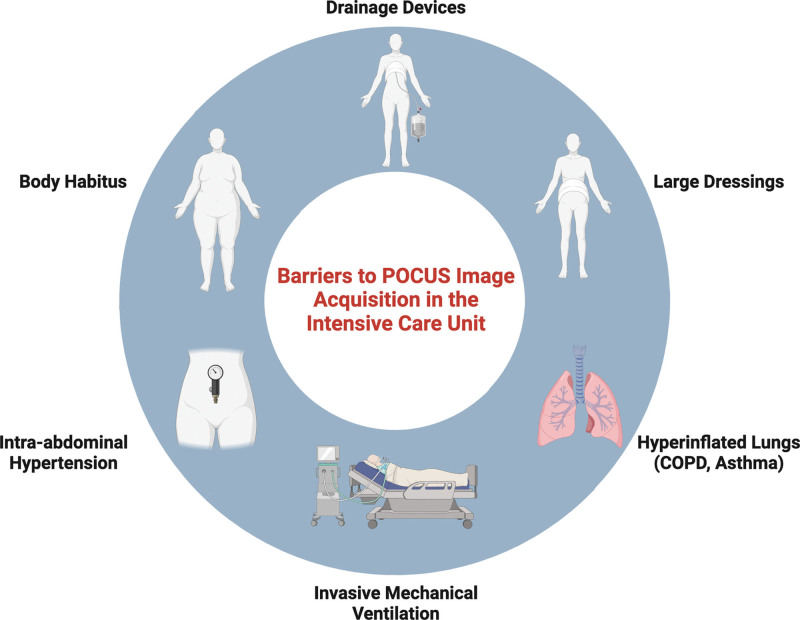
Point-of-care ultrasonography (POCUS) is an imaging modality used in the emergency department, wards, preoperative and postanesthesia care unit, and ICU settings that provides essential information for rapid real-time diagnosis as well as assessment of treatment responses in patients with life-threatening diseases. As such, POCUS has become the standard of care for use in caring for the critically ill (1–4). However, proper POCUS interpretation requires adequate transthoracic image acquisition. Impediments to transthoracic and abdominal echocardiographic windows often exist in critically ill patients due to body habitus, intra-abdominal hypertension, dressings or drainage tubes, postoperative sternotomies, invasive mechanical ventilation, the presence of subcutaneous emphysema, or lung hyperinflation (5) (Fig. 1). This narrative review will focus on point-of-care echocardiography in difficult-to-image patients and aims to highlight common problems encountered in the ICU followed by techniques to optimize image acquisition to acquire interpretable images by overcoming patient-specific factors or incorporating alternative views into a POCUS examination (Fig. 2).
Figure 1.
Schematic illustrating commonly encountered obstacles to transthoracic echocardiography. COPD = chronic obstructive pulmonary disease. Created with Biorender.com.
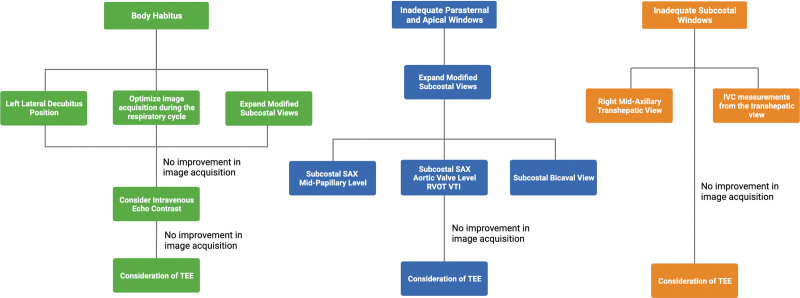
Figure 2.
Schematic illustrating maneuvers to improve image acquisition by specific obstacles. IVC = inferior vena cava, RVOT VTI = velocity time integral of the right ventricular outflow tract, SAX = short axis, TEE = transesophageal echocardiography.
BODY HABITUS
Obesity is highly prevalent in the ICU setting, affecting an estimated 20% of ICU patients (6), and may result in inadequate transthoracic images for bedside interpretation, particularly as weight exceeds 250–300 lbs (7). Ultrasound waves are attenuated by adipose tissue, thereby reducing image quality in obese patients. Additionally, there is often increased distance between the ultrasound transducer and heart. To improve image acquisition and quality, the bedside clinician can make some adjustments to help overcome some of these challenges. These adjustments rely on patient position, contrast administration, modified conventional POCUS views, and breath holds.
Patient positioning can play an important role in transthoracic echocardiography (TTE). Repositioning critically ill patients may be challenging for providers due to patient safety, particularly in the presence of obesity, an inability of the patient to assist, the presence of indwelling invasive devices, and other impediments. The left lateral decubitus position has been shown to improve parasternal and apical views by more than 12% by bringing the heart closer to the chest wall (8, 9). In obese patients, this will decrease the depth of penetration required for the ultrasound waves and ultimately decrease the amount of ultrasound wave attenuation. Repositioning often requires multiple providers and unilateral wedges placed under the patient can assist in achieving left lateral decubitus positioning. However, despite optimizing patient positioning, some obese patients will continue to have inadequate parasternal and apical windows. IV microbubble echo contrast has been shown to improve endocardial border opacification in critically ill patients with difficult transthoracic windows, allowing for improved evaluation of left ventricular (LV) function (5, 10, 11). However, IV echo contrast may not be readily available at most institutions for POCUS examinations. Alternatively, some obese patients may have adequate views from the subcostal window when compared with the parasternal and apical windows. Additional advanced echocardiographic assessments can be made by modifying the subcostal views which will be discussed further below. Subcostal views may also be improved by bending the patient’s knees enhancing abdominal muscle relaxation. A subset of patients may have transient quality images in the subcostal window throughout the respiratory cycle. During inspiration, the heart moves inferiorly toward the ultrasound transducer when placed in the subcostal position. If a patient is able to cooperate, the clinician may request a patient perform a full breath hold to improve subcostal image quality; alternatively, in mechanically ventilated patients, an inspiratory hold maneuver can be performed in an effort to improve image quality (12). Finally, while adequate image quality may still be challenging secondary to body habitus, presence of bowel gas, and postoperative states, some patients may still have obtainable dynamic assessments such as tricuspid annular plane systolic excursion (TAPSE), mitral annular plane systolic excursion or tissue Doppler imaging S’ that may provide valuable information about right ventricular (RV) and LV functions.
INADEQUATE PARASTERNAL AND APICAL WINDOWS
The use of TTE in the assessment of cardiac function and hemodynamics in critically ill patients is largely performed using the parasternal and apical windows. However, many patients in the ICU setting have impediments to adequate image acquisition at these locations (10, 13). Invasive mechanical ventilation and lung hyperinflation (as seen in chronic obstructive pulmonary disease and asthma) often interpose aerated lung parenchyma between the ultrasound transducer and heart leading to scattering and reflection of the ultrasound waves, low acoustic impedance, and a high attenuation coefficient (14). This may translate to visualization of reverberation artifacts with the pleural line and lung parenchyma. Patients receiving mechanical ventilation may have difficult parasternal or apical images as the addition of positive end-expiratory pressure may displace the heart inferiorly due to diaphragmatic flattening (15). Postoperative patients after thoracotomies or sternotomies may also have dressings and/or drainage tubes that obscure the parasternal or apical windows. Patients undergoing cardiopulmonary resuscitation (CPR) also often have inadequate parasternal views due to positioning of defibrillator pads and ongoing manual or mechanical chest compressions. In the absence of parasternal and apical views, the bedside clinician can gain additional information about cardiac performance by incorporating modified subcostal views.
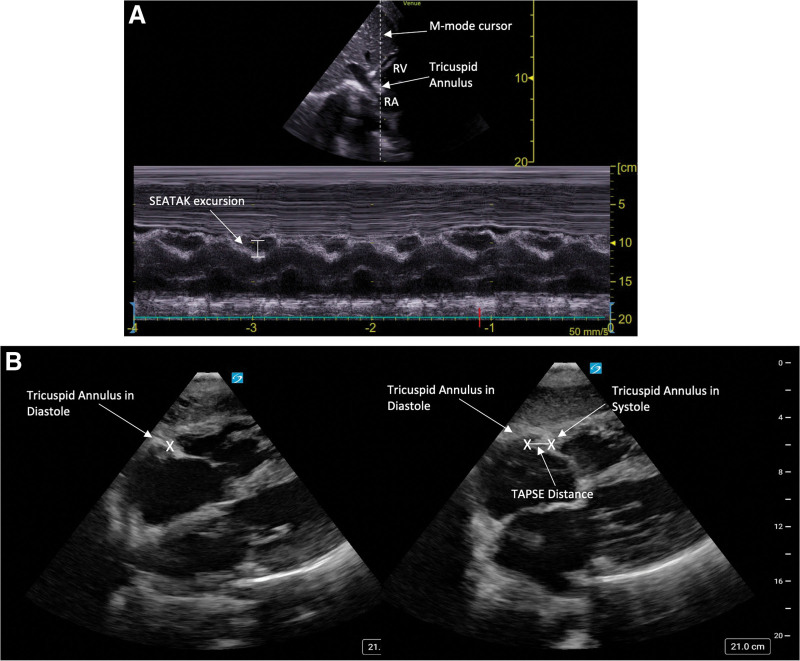
Traditional subcostal views include the subcostal 4 and 5 chamber views and the subcostal inferior vena cava (IVC) view (eFig. 1A–C, http://links.lww.com/CCX/B295) (16, 17). These views provide vital information about the presence of pericardial pathology, global biventricular function, atrial pathology, valvulopathy, estimation of right atrial pressure, intravascular volume status, and volume responsiveness (15, 18–21). Qualitative assessments of RV and LV size and function and the presence of a pericardial effusion have shown strong agreement between subcostal and parasternal/apical views (22). In addition to traditional measurements taken from the subcostal window, advanced echocardiographic assessments may also be performed from this window in the absence of adequate parasternal and/or apical windows. For example, although base-to-apex RV function is often evaluated using TAPSE from the traditional apical four-chamber view (23, 24), the subcostal echocardiographic assessment of tricuspid annular kick can be used to evaluate RV systolic function from the subcostal window (25). This is similarly accomplished by placing M-mode over the tricuspid annulus and measuring excursion (Fig. 3A). Due to the change in orientation from the subcostal view, lower values are expected in normal RV function (about 2–3 mm lower than TAPSE values), with a value of greater than or equal to 1.6 cm indicative of normal RV function (25, 26). Alternatively, one can identify and mark the tricuspid annulus in systole and diastole and measure the distance between the two points, which has been shown to correlate well with traditional TAPSE measurements performed from the apical window (Fig. 3B) (27).
Figure 3.
Point-of-care ultrasonography (POCUS) images displaying: (A) Calculation of subcostal echocardiographic assessment of tricuspid annular kick (SEATAK) from the subcostal short-axis (SAX) view, which is calculated by placing M-mode over the tricuspid annulus in a SAX view from the subcostal window and measuring excursion, (B) Estimation of tricuspid annular plane systolic excursion (TAPSE) from the subcostal four-chamber view by measuring the distance between the tricuspid annulus in diastole and systole. RA = right atrium, RV = right ventricle.
The subcostal window can also be modified to obtain short-axis (SAX) cardiac views, providing additional information about cardiac performance in multiple planes. For example, the subcostal SAX view at the midpapillary level may be obtained by tilting the probe more cephalad from the subcostal IVC view (increasing the window into the thorax), and by fanning toward the right flank (Fig. 4). Just as with the parasternal SAX view, this view can be used to evaluate global LV function, including advanced echocardiographic assessments such as fractional area change, signs of RV dilation, septal flattening during end-systole and/or end-diastole to evaluate RV volume and/or pressure overload, as well as regional wall motion abnormalities (eFig. 2, A and B, http://links.lww.com/CCX/B295) (15).
Figure 4.
Schematic illustrating angle of insonation and fanning direction to obtain subcostal SAX views. Arrows indicate direction of fanning moving from papillary muscle level to aortic valve level. Created with Biorender.com.
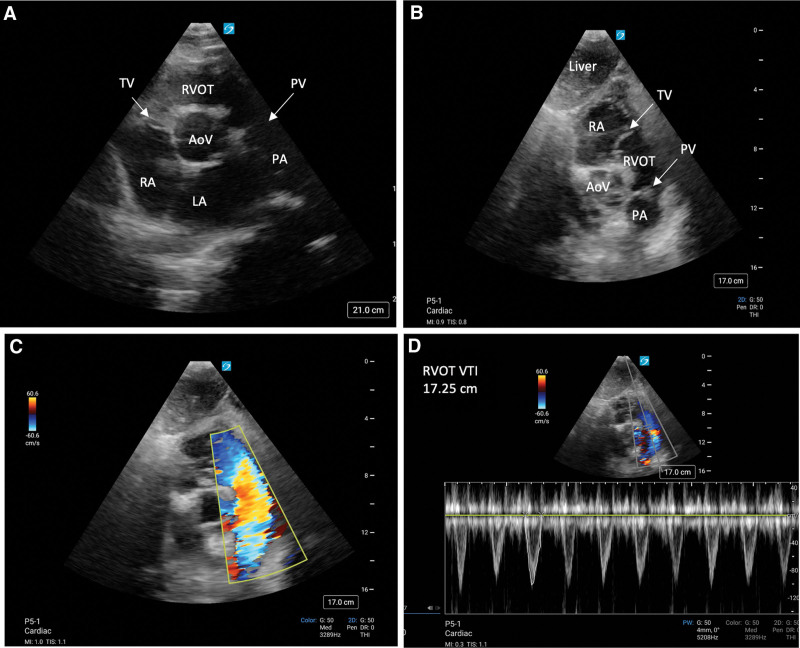
The subcostal SAX view at the level of the aortic valve and RV outflow tract (RVOT) can also be obtained by fanning toward the right shoulder from the midpapillary level (Fig. 5, A and B). Assessment of the aortic, tricuspid, and pulmonary valves can be obtained from this view, including color flow Doppler to evaluate for valvular regurgitation and estimation of pulmonary artery pressures. Hemodynamic assessments may also be performed using the velocity time integral of the RVOT (RVOT VTI) (Fig. 5, C and D). Although traditionally the LV outflow tract (LVOT) VTI has been used to estimate stroke volume and ultimately cardiac output and cardiac index using the equation , where SV is the stroke volume, DLVOT is the diameter of the LVOT measured in the parasternal long axis, and VTILVOT is the LVOT VTI (16). However, in the absence of adequate views to obtain LVOT VTI Doppler assessment from the apical window, RVOT VTI may be used as an alternative measurement to infer stroke volume. Although the cutoff for normal LVOT VTI is greater than 17 cm (28, 29), the average RVOT diameter is both slightly larger and structurally different than that of the LVOT, and its cross-sectional area cannot be as readily determined in the same manner as for the LVOT. Therefore, the cutoff for normal is slightly lower for RVOT VTI, generally greater than 14 cm (30, 31). However, evidence suggests there is moderate agreement in comparing the RVOT VTI obtained from a subcostal SAX view and LVOT VTI in critically ill patients, and this view was obtained in 90% of patients (32). RVOT VTI has also been shown to correlate to cardiac index, with a cutoff of less than 9.5 cm highly predictive of cardiac index less than 2.2 L/min/m2 and associated with increased mortality in critically ill patients with pulmonary embolism (33). Although directly estimating SV, cardiac output, and cardiac index using RVOT VTI may be challenging, RVOT VTI measured from the subcostal view can be used as a screening tool to identify patients who may be in low SV states. The use of RVOT VTI may be less reliable in patients for whom RV and LV stroke volume differ, for example, those with intracardiac shunts or severe valvular regurgitation.
Figure 5.
Point-of-care ultrasonography (POCUS) images displaying: (A) parasternal short-axis (SAX) aortic valve (AoV)/right ventricular outflow tract (RVOT) view, (B) subcostal SAX AoV/RVOT view, (C) subcostal SAX AoV/RVOT view with color-flow Doppler displaying flow away from the transducer through the RVOT, (D) calculation of RVOT velocity time integral (VTI) from the subcostal SAX AoV/RVOT view. LA = left atrium, PA = pulmonary artery, PV = pulmonic valve, RA = right atrium, RV = right ventricle, TV = tricuspid valve.
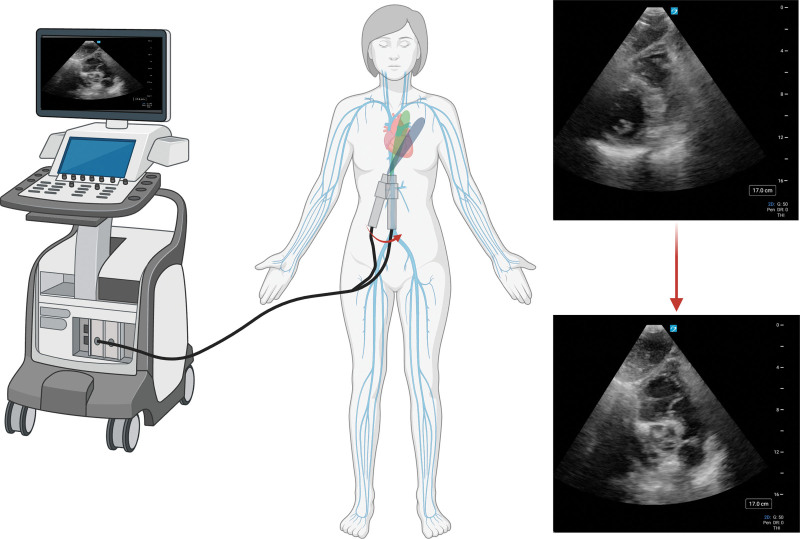
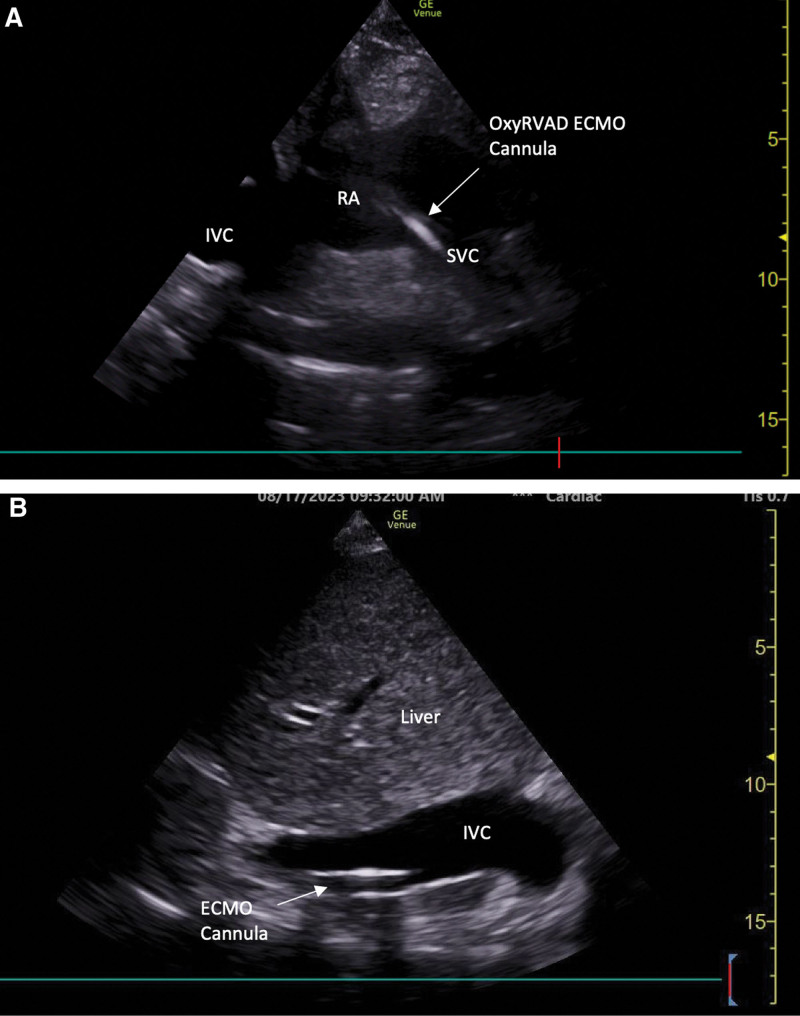
The bedside clinician can also enhance the POCUS examination from the subcostal region by obtaining the subcostal bicaval view by tilting the probe more cephalad from the traditional sagittal IVC view (Fig. 6A). This view may be of particular interest in the setting of determining cannula positioning during extracorporeal life support or superior vena cava pathology (15).
Figure 6.
Point-of-care ultrasonography (POCUS) images displaying: (A) subcostal bicaval view, (B) right midaxillary line transhepatic coronal inferior vena cava (IVC) view. ECMO = extracorporeal membrane oxygenation, LA = left atrium, LV = left ventricle, LVOT = left ventricular outflow tract, OxyRVAD = right ventricular assist device with membrane oxygenator, RA = right atrium, RV = right ventricle, SVC = superior vena cava.
In the specific case of POCUS during CPR, the subcostal region provides an optimal window to identify causes of pulseless electrical activity (specifically right heart strain and pericardial tamponade) (34, 35), presence of cardiac activity which has been associated with improved return of spontaneous circulation and survival to hospital discharge (36), and to monitor the adequate positioning of chest compressions (34). Furthermore, the use of the subcostal window has been demonstrated to be feasible in providing adequate and interpretable images during CPR pulse checks without prolonging no-flow time between CPR cycles (34, 35).
INADEQUATE SUBCOSTAL WINDOWS: THE INFERIOR VENA CAVA
The subcostal sagittal view of the IVC has become an essential component of the POCUS examination in critically ill patients. It can provide vital information regarding intravascular volume status and estimation of right atrial pressure in select patients, and while it has traditionally been used for evaluation of volume responsiveness prediction, accurate prediction using IVC can be fraught with challenges and there is significant heterogeneity in the literature regarding its use (18–21, 37–41). It may be more reasonable to use IVC dimensions as well as the extremes of its collapsibility or distensibility index to improve diagnostic accuracy to rule in/out a patient who may be volume responsive. For example, in one study, a cutoff of IVC collapsibility index in spontaneous breathing patients of 42% was 97% specific and only 31% sensitive for predicting fluid responsiveness; however, a cutoff of 20% would have improved sensitivity to 79% at the expense of specificity (42). Therefore, it is certainly worthwhile to include IVC measurements in the global context of the point-of-care echocardiogram. However, subcostal windows may be inaccessible in ICU patients in the setting of dressings, drains, and body habitus. In this scenario, if the right midaxillary window is accessible, a transhepatic view of the IVC in the coronal plane can be obtained (Fig. 6B). It should be noted that these measurements may not be interchangeable. A number of small single-center studies have been performed comparing subcostal and transhepatic assessments of the IVC as it relates to size, distensibility or collapsibility, and prediction of volume responsiveness (43–48). There is varying agreement amongst these studies, likely due to the nature of the IVC collapsing as an elliptical shape (43, 49). One study found that an IVC collapsibility index of 42% should be used to predict fluid responsiveness when using the transhepatic IVC view in spontaneously breathing patients (48). Though the transhepatic view of the IVC can be used in the absence of accessible subcostal windows, caution should be used when interpreting measurements at the bedside. Additionally, in patients with intra-abdominal hypertension, the IVC becomes a poor surrogate of intravascular volume status and volume responsiveness due to extrinsic compression and decreased venous return (50).
FUTURE DIRECTIONS: POINT-OF-CARE TRANSESOPHAGEAL ECHO
Despite all the maneuvers described above, there will still be a small subset of critically ill patients in the ICU who will not have any meaningful interpretable image for diagnostic and therapeutic interpretation. These patients, particularly those who are already sedated and mechanically ventilated, may benefit from point-of-care transesophageal echocardiography (TEE) to obtain vital information regarding cardiac and hemodynamic performance. Point-of-care TEE is feasible and safe in critically ill patients, although requires specific training and competence of the sonographer (51–53). TEE provides high-quality images without the impediments experienced with TTE. In addition, transesophageal echo is also advantageous in several scenarios. Postoperative cardiac surgery patients with sternotomy wounds and chest wall devices benefit from transesophageal echo in the setting of unexplained hypotension (54), particularly as localized cardiac tamponade, for example, a posterior pericardial hematoma with right atrial compression, may not be readily identified, even with adequate transthoracic views. Just as with TTE, TEE can also provide information about preload sensitivity and provides a superior view of the superior vena cava which can be used to assess volume responsiveness (55). In addition, TEE provides increased sensitivity for the evaluation of intracardiac shunts and valvular disorders, including vegetations, when compared with TTE (51). Other advantages of TEE over TTE, particularly in the presence of inadequate transthoracic windows, include identification of the cause of cardiac arrest during CPR (56), identification of aortic dissection and central pulmonary embolus, as well as real-time procedural guidance such as during extracorporeal membrane oxygenation cannulation, percutaneous RV assist device placement, and intra-aortic balloon pump placement (51). Given some of the limitations of TTE and additional benefits of TEE, there is increasing demand for training in TEE competency amongst general intensivists. Evidence suggests that point-of-care TEE in the ICU improves diagnostic yield beyond TTE, often changes management, and basic midesophageal and transgastric views are readily learned by trainees (52, 57). However, TEE does not come without risks. Contraindications include significant esophageal disease such as stricture or recent esophageal or gastric surgery. Severe complications may include oropharyngeal bleeding, esophageal bleeding, and esophageal perforation, although these are rare complications and occur in roughly 0.01–0.5% of cases (57, 58).
CONCLUSIONS
Bedside echocardiography provides vital information pertaining to rapid diagnoses and responses to therapeutic interventions for critically ill patients in the ICU. However, the bedside clinician may face several impediments to obtaining quality transthoracic images, namely body habitus, the presence of wounds, dressings, and drainage tubes, invasive mechanical ventilation, intra-abdominal hypertension, and hyperinflated lungs. A stepwise approach can be taken to improve image quality including patient positioning, timing of image acquisition during the respiratory cycle, the use of modified views from the subcostal window, the use of transhepatic coronal imaging for IVC assessment, administration of contrast, and ultimately point-of-care TEE in select patients (Fig. 2). Clinicians should use caution when interpreting modified views as further research is needed in these areas to correlate to advanced echocardiographic measurements obtained from traditional transthoracic parasternal and apical views.
Supplementary Material
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccejournal).
Dr. Grotberg received funding from the National Heart, Lung, and Blood Institute (T32 HL007317). The remaining authors have not disclosed that they do not have any potential conflicts of interest.
All authors participated in the article development. Dr. Grotberg drafted the article, which was critically reviewed by McDonald and Co. All authors approved of the final article.
REFERENCES
- 1.Moore CL, Copel JA: Point-of-care ultrasonography. N Engl J Med 2011; 364:749–757 [DOI] [PubMed] [Google Scholar]
- 2.Via G, Hussain A, Wells M, et al. ; International Liaison Committee on Focused Cardiac UltraSound (ILC-FoCUS): International evidence-based recommendations for focused cardiac ultrasound. J Am Soc Echocardiogr 2014; 27:683.e1–683.e33 [DOI] [PubMed] [Google Scholar]
- 3.Frankel HL, Kirkpatrick AW, Elbarbary M, et al. : Guidelines for the appropriate use of bedside general and cardiac ultrasonography in the evaluation of critically ill patients-part I: General ultrasonography. Crit Care Med 2015; 43:2479–2502 [DOI] [PubMed] [Google Scholar]
- 4.Levitov A, Frankel HL, Blaivas M, et al. : Guidelines for the appropriate use of bedside general and cardiac ultrasonography in the evaluation of critically ill patients—part II: Cardiac ultrasonography. Crit Care Med 2016; 44:1206–1227 [DOI] [PubMed] [Google Scholar]
- 5.Cosyns B, El Haddad P, Lignian H, et al. : Contrast harmonic imaging improves the evaluation of left ventricular function in ventilated patients: Comparison with transesophageal echocardiography. Eur J Echocardiogr 2004; 5:118–122 [DOI] [PubMed] [Google Scholar]
- 6.Schetz M, De Jong A, Deane AM, et al. : Obesity in the critically ill: A narrative review. Intensive Care Med 2019; 45:757–769 [DOI] [PubMed] [Google Scholar]
- 7.Uppot RN: Impact of obesity on radiology. Radiol Clin North Am 2007; 45:231–246 [DOI] [PubMed] [Google Scholar]
- 8.Mitchell C, Rahko PS, Blauwet LA, et al. : Guidelines for performing a comprehensive transthoracic echocardiographic examination in adults: Recommendations from the American Society of Echocardiography. J Am Soc Echocardiogr 2019; 32:1–64 [DOI] [PubMed] [Google Scholar]
- 9.Ottenhoff J, Hewitt M, Makonnen N, et al. : Comparison of the quality of echocardiography imaging between the left lateral decubitus and supine positions. Cureus. 2022; 14:6–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nguyen TT, Dhond MR, Sabapathy R, et al. : Contrast microbubbles improve diagnostic yield in ICU patients with poor echocardiographic windows. Chest 2001; 120:1287–1292 [DOI] [PubMed] [Google Scholar]
- 11.Shah BN, Senior R: Stress echocardiography in patients with morbid obesity. Echo Res Pract 2016; 3:R13–R18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ginghina C, Beladan CC, Iancu M, et al. : Respiratory maneuvers in echocardiography: A review of clinical applications. Cardiovasc Ultrasound 2009; 7:1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lazzeri C, Cianchi G, Bonizzoli M, et al. : The potential role and limitations of echocardiography in acute respiratory distress syndrome. Ther Adv Respir Dis. 2016; 10:136–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bakhru RN, Schweickert WD: Intensive care ultrasound: I Physics, equipment, and image quality. Ann Am Thorac Soc. 2013; 10:540–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Flower L, Madhivathanan PR, Andorka M, et al. : Getting the most from the subcostal view: The rescue window for intensivists. J Crit Care 2021; 63:202–210 [DOI] [PubMed] [Google Scholar]
- 16.Narasimhan M, Koenig SJ, Mayo PH: Advanced echocardiography for the critical care physician: Part 1. Chest 2014; 145:129–134 [DOI] [PubMed] [Google Scholar]
- 17.Narasimhan M, Koenig SJ, Mayo PH: Advanced echocardiography for the critical care physician: Part 2. Chest 2014; 145:129–134 [DOI] [PubMed] [Google Scholar]
- 18.Barbier C, Schmit C, Ricôme J, et al. : Respiratory changes in inferior vena cava diameter are helpful in predicting fluid responsiveness in ventilated septic patients. Intensive Care Med 2004; 30:1740–1746 [DOI] [PubMed] [Google Scholar]
- 19.Miller A, Mandeville J: Predicting and measuring fluid responsiveness with echocardiography. Echo Res Pract 2016; 3:G1–G12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Corl KA, George NR, Romanoff J, et al. : Inferior vena cava collapsibility detects fluid responsiveness among spontaneously breathing critically-ill patients. J Crit Care 2017; 41:130–137 [DOI] [PubMed] [Google Scholar]
- 21.Vignon P, Repessé X, Begot E, et al. : Comparison of echocardiographic indices used to predict fluid responsiveness in ventilated patients. Am J Respir Crit Care Med 2017; 195:1022–1032 [DOI] [PubMed] [Google Scholar]
- 22.Bughrara N, Renew JR, Alabre K, et al. : Comparison of qualitative information obtained with the echocardiographic assessment using subcostal-only view and focused transthoracic echocardiography examinations: A prospective observational study. Can J Anaesth 2022; 69:196–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rudski LG, Lai WW, Afilalo J, et al. : Guidelines for the echocardiographic assessment of the right heart in adults: A report from the American Society of Echocardiography Endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and. J Am Soc Echocardiogr 2010; 23:685–713; quiz 786 [DOI] [PubMed] [Google Scholar]
- 24.Daley J, Grotberg J, Pare J, et al. : Emergency physician performed tricuspid annular plane systolic excursion in the evaluation of suspected pulmonary embolism. Am J Emerg Med 2017; 35:106–111 [DOI] [PubMed] [Google Scholar]
- 25.Díaz-Gómez J, Borja Alvarez A, Danaraj J, et al. : A novel semiquantitative assessment of right ventricular systolic function with a modified subcostal echocardiographic view. Echocardiogr 2017; 34:44–52 [DOI] [PubMed] [Google Scholar]
- 26.Sadek D, Al-Deftar M, Attia W, et al. : Value of semiquantitative assessment of right ventricular systolic function with a modified subcostal echocardiographic view. Egypt J Hosp Med 2019; 75:2601–2605 [Google Scholar]
- 27.Main AB, Braham R, Campbell D, et al. : Subcostal TAPSE: A retrospective analysis of a novel right ventricle function assessment method from the subcostal position in patients with sepsis. Ultrasound J 2019; 11:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blanco P: Rationale for using the velocity–time integral and the minute distance for assessing the stroke volume and cardiac output in point-of-care settings. Ultrasound J 2020; 12:1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goldman JH, Schiller NB, Lim DC, et al. : Usefulness of stroke distance by echocardiography as a surrogate marker of cardiac output that is independent of gender and size in a normal population. Am J Cardiol 2001; 87:499–502, A8 [DOI] [PubMed] [Google Scholar]
- 30.Choi JO, Shin MS, Kim MJ, et al. : Normal echocardiographic measurements in a Korean population study: Part II Doppler and tissue Doppler imaging. J Cardiovasc Ultrasound. 2016; 24:144–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mercadal J, Borrat X, Hernández A, et al. ; Spanish Critical Care Ultrasound Network Group: A simple algorithm for differential diagnosis in hemodynamic shock based on left ventricle outflow tract velocity–time integral measurement: A case series. Ultrasound J 2022; 14:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Colinas Fernández L, Hernández Martínez G, Serna Gandía MB, et al. : Improving echographic monitoring of hemodynamics in critically ill patients: Validation of right cardiac output measurements through the modified subcostal window. Med Intensiva 2023; 47:149–156 [DOI] [PubMed] [Google Scholar]
- 33.Brailovsky Y, Lakhter V, Weinberg I, et al. : Right ventricular outflow Doppler predicts low cardiac index in intermediate risk pulmonary embolism. Clin Appl Thromb 2019; 25:107602961988606–107602961988607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gardner KF, Clattenburg EJ, Wroe P, et al. : The Cardiac Arrest Sonographic Assessment (CASA) exam—a standardized approach to the use of ultrasound in PEA. Am J Emerg Med 2018; 36:729–731 [DOI] [PubMed] [Google Scholar]
- 35.Bughrara N, Herrick S, Leimer E, et al. : Focused cardiac ultrasound and the periresuscitative period: A case series of resident-performed echocardiographic assessment using subcostal-only view in advanced life support. A A Pract. 2020; 14:e01278. [DOI] [PubMed] [Google Scholar]
- 36.Gaspari R, Weekes A, Adhikari S, et al. : Emergency department point-of-care ultrasound in out-of-hospital and in-ED cardiac arrest. Resuscitation 2016; 109:33–39 [DOI] [PubMed] [Google Scholar]
- 37.Feissel M, Michard F, Faller JP, et al. : The respiratory variation in inferior vena cava diameter as a guide to fluid therapy. Intensive Care Med 2004; 30:1834–1837 [DOI] [PubMed] [Google Scholar]
- 38.Moreno FLL, Hagan AD, Holmen JR, et al. : Evaluation of size and dynamics of the inferior vena cava as an index of right-sided cardiac function. Am J Cardiol 1984; 53:579–585 [DOI] [PubMed] [Google Scholar]
- 39.Di Nicolò P, Tavazzi G, Nannoni L, et al. : Inferior vena cava ultrasonography for volume status evaluation: An intriguing promise never fulfilled. J Clin Med 2023; 12:1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Via G, Tavazzi G, Price S: Ten situations where inferior vena cava ultrasound may fail to accurately predict fluid responsiveness: A physiologically based point of view. Intensive Care Med 2016; 42:1164–1167 [DOI] [PubMed] [Google Scholar]
- 41.Orso D, Paoli I, Piani T, et al. : Accuracy of ultrasonographic measurements of inferior vena cava to determine fluid responsiveness: A systematic review and meta-analysis. J Intensive Care Med 2020; 35:354–363 [DOI] [PubMed] [Google Scholar]
- 42.Airapetian N, Maizel J, Alyamani O, et al. : Does inferior vena cava respiratory variability predict fluid responsiveness in spontaneously breathing patients? Crit Care 2015; 19:1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.La Via L, Astuto M, Dezio V, et al. : Agreement between subcostal and transhepatic longitudinal imaging of the inferior vena cava for the evaluation of fluid responsiveness: A systematic review. J Crit Care 2022; 71:1–8 [DOI] [PubMed] [Google Scholar]
- 44.Kulkarni AP, Janarthanan S, Harish MM, et al. : Agreement between inferior vena cava diameter measurements by subxiphoid versus transhepatic views. Indian J Crit Care Med. 2015; 19:719–722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Garijo JM, Wijeysundera DN, Munro JC, et al. : Correlation between transhepatic and subcostal inferior vena cava views to assess inferior vena cava variation: A pilot study. J Cardiothorac Vasc Anesth 2017; 31:973–979 [DOI] [PubMed] [Google Scholar]
- 46.Valette X, Ribstein P, Ramakers M, et al. : Subcostal versus transhepatic view to assess the inferior vena cava in critically ill patients. Echocardiogr 2020; 37:1171–1176 [DOI] [PubMed] [Google Scholar]
- 47.Yao B, Liu JY, Sun YB, Zhao YX, Li L-di: The value of the inferior vena cava area distensibility index and its diameter ratio for predicting fluid responsiveness in mechanically ventilated patients. Shock 2019; 52:37–42 [DOI] [PubMed] [Google Scholar]
- 48.Shah R, Spiegel R, Lu C, et al. : Relationship between the subcostal and right lateral ultrasound views of inferior vena cava collapse: Implications for clinical use of ultrasonography. Chest 2018; 153:939–945 [DOI] [PubMed] [Google Scholar]
- 49.Finnerty NM, Panchal AR, Boulger C, et al. : Inferior vena cava measurement with ultrasound: What is the best view and best mode? West J Emerg Med. 2017; 18:496–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bauman Z, Coba V, Gassner M, et al. : Inferior vena cava collapsibility loses correlation with internal jugular vein collapsibility during increased thoracic or intra-abdominal pressure. J Ultrasound 2015; 18:343–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mayo PH, Narasimhan M, Koenig S: Critical care transesophageal echocardiography. Chest 2015; 148:1323–1332 [DOI] [PubMed] [Google Scholar]
- 52.Garcia YA, Quintero L, Singh K, et al. : Feasibility, safety, and utility of advanced critical care transesophageal echocardiography performed by pulmonary/critical care fellows in a medical ICU. Chest 2017; 152:736–741 [DOI] [PubMed] [Google Scholar]
- 53.Jaidka A, Hobbs H, Koenig S, et al. : Better with ultrasound: Transesophageal echocardiography. Chest 2019; 155:194–201 [DOI] [PubMed] [Google Scholar]
- 54.Prager R, Ainsworth C, Arntfield R: Critical care transesophageal echocardiography for the resuscitation of shock: An important diagnostic skill for the modern intensivist. Chest 2023; 163:268–269 [DOI] [PubMed] [Google Scholar]
- 55.Vieillard-Baron A, Chergui K, Rabiller A, et al. : Superior vena caval collapsibility as a gauge of volume status in ventilated septic patients. Intensive Care Med 2004; 30:1734–1739 [DOI] [PubMed] [Google Scholar]
- 56.Rosseel T, Van Puyvelde T, Voigt JU, et al. : How to perform focused transoesophageal echocardiography during extracorporeal cardiopulmonary resuscitation? Eur Heart J Cardiovasc Imaging. 2023; 24:12–14 [DOI] [PubMed] [Google Scholar]
- 57.Prager R, Bowdridge J, Pratte M, et al. : Indications, clinical impact, and complications of critical care transesophageal echocardiography: A scoping review. J Intensive Care Med 2023; 38:245–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hilberath JN, Oakes DA, Shernan SK, et al. : Safety of transesophageal echocardiography. J Am Soc Echocardiogr 2010; 23:1115–27; quiz 1220 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.