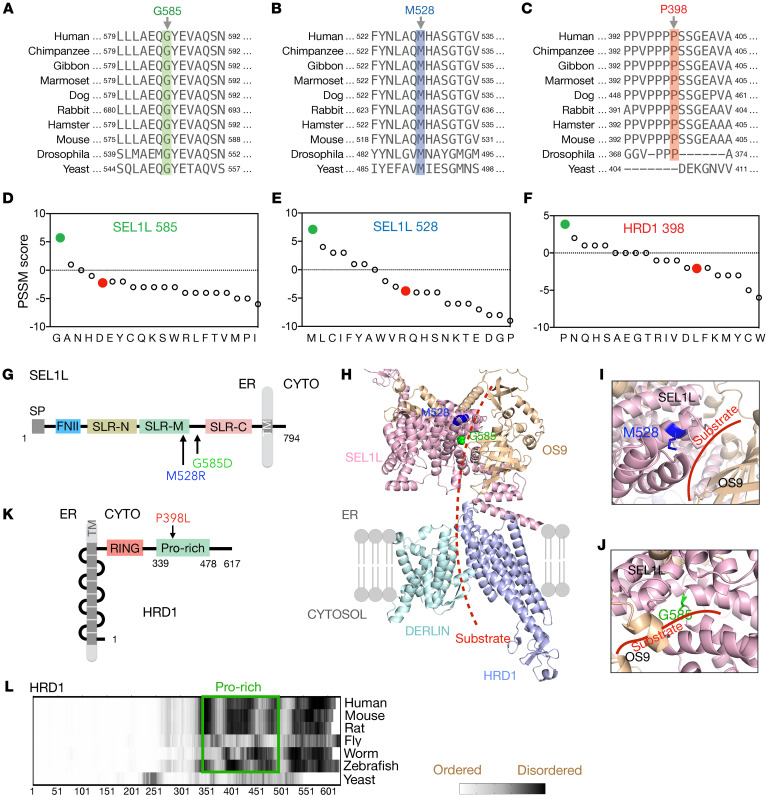
Figure 4. Sequence and structural analyses of SEL1L and HRD1 variants.
(A–C) The aa sequence alignment of SEL1L (A and B) and HRD1 (C) showing the conservation of residues across species. (D–F) PSSM scores for aa position in SEL1L (D and E) and HRD1 proteins (F), with WT in green and variants in red. (G–K) Schematic diagrams of human SEL1L (G) and HRD1 (K) with the location of the variants indicated. SP, signal peptide; FNII, fibronectin type II domain; SLR-N/M/C, Sel1-like repeats at N-terminal, middle-, and C-terminal; TM, transmembrane; CYTO, cytosol; RING, RING domain; Pro-rich, Proline-rich domain. (H–J) Structural prediction of human SEL1L/OS9/HRD1/DERLIN ERAD complex using AlphaFold2 with close-up views of SEL1L-M528 (blue) and G585 (green) areas shown in I and J. Red (dotted) line marks the putative substrate binding groove. (L) Comparison of disordered region of HRD1 across species, highlighting the disordered nature of the proline-rich domain.