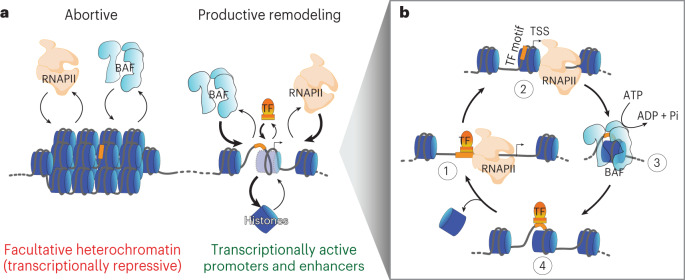
Fig. 6. RNAPII, BAF and DNA-sequence-specific TFs work synergistically in a dynamic cycle for productive chromatin remodeling and nucleosome eviction.
a, Model showing that RNAPII and BAF dynamically engage chromatin in an abortive manner (left-hand side) and require chromatin binding by DNA-sequence-specific TFs for productive chromatin remodeling and histone eviction to form/maintain an NDR (right-hand side). Relative thickness of arrows implies enrichment of factor-bound states in transcriptionally active chromatin as observed in steady-state bulk measurement. b, Steady-state promoter and enhancer chromatin structures can be explained by a dynamic cycle of nucleosome deposition and eviction and synergistic RNAPII, BAF and TF activity. We speculate that the cycle can start at any step: RNAPII loading at nucleosome-depleted regions (step 1) and transcription initiation (step 2), BAF binding to nucleosomes (step 3) or TF-binding nucleosomes that are partially unwrapped due to spontaneous thermal fluctuations in histone–DNA interactions or BAF binding and remodeling (step 4); and the cycle can continue as long as factor concentrations are high enough. The steps in the cycle facilitate each other, which we propose based on our observations that RNAPII promoter-proximal pausing promotes BAF occupancy and ATP-dependent nucleosome eviction, BAF is associated with partially unwrapped nucleosomes at pluripotency TF-binding sites and upregulated TF protein expression promotes nucleosome eviction by BAF leading to stable NDR formation, which facilitates new RNAPII loading. The RNAPII illustration was created with BioRender.com. Pi, inorganic phosphate.