Abstract
Betaine, a compound found in plants and sea foods, is known to be beneficial against non-alcoholic fatty liver disease (NAFLD), but its hepatoprotective and anti-steatogenic mechanisms have been not fully understood. In the present study, we investigated the mechanisms underlying betaine-mediated alleviation of NAFLD induced by a choline-deficient, L-amino acid-defined, high-fat diet (CDAHFD) in mice, with special focus on the contribution of betaine-stimulated autophagy to NAFLD prevention. Male ICR mice were fed a CDAHFD with or without betaine (0.2–1% in drinking water) for 1 week. Betaine ameliorated the CDAHFD-induced fatty liver by restoring sulfur amino acid (SAA)-related metabolites, such as S-adenosylmethionine and homocysteine, and the phosphorylation of AMPK and ACC. In addition, it reduced the CDAHFD-induced ER stress (BiP, ATF6, and CHOP) and apoptosis (Bax, cleaved caspase-3, and cleaved PARP); however, it induced autophagy (LC3II/I and p62) which was downregulated by CDAHFD. To determine the role of autophagy in the improvement of NAFLD, chloroquine (CQ), an autophagy inhibitor, was injected into the mice fed a CDAHFD and betaine (0.5 % in drinking water). CQ did not affect SAA metabolism but reduced the beneficial effects of betaine as shown by the increases of hepatic lipids, ER stress, and apoptosis. Notably, the betaine-induced improvements in lipid metabolism determined by protein levels of p-AMPK, p-ACC, PPARα, and ACS1, were reversed by CQ. Thus, the results of this study suggest that the activation of autophagy is an important upstream mechanism for the inhibition of steatosis, ER stress, and apoptosis by betaine in NAFLD.
Keywords: Betaine, Non-alcoholic fatty liver disease, Autophagy, S-amino acid metabolism, Endoplasmic reticulum stress, Apoptosis
Graphical abstract
Highlights
-
•
Betaine attenuates choline-deficient, amino acid-defined, high-fat diet-induced NAFLD.
-
•
Betaine restores the dysregulation of hepatic S-amino acid metabolism in NAFLD.
-
•
Betaine improves hepatic autophagy, steatosis, ER stress, and apoptosis in NAFLD.
-
•
Inhibition of autophagy by chloroquine suppresses betaine-promoted hepatoprotection.
-
•
Betaine protects the liver from CDAHFD-induced NAFLD.
Abbreviations
- ACC
Acetyl-CoA carboxylase
- ACS1
Acyl-CoA synthetase 1
- ALT
Alanine transaminase
- AMPK
AMP-activated protein kinase
- ANOVA
Analysis of variation
- ATF6
activating transcription factor 6
- Atg7
Autophagy related 7
- Bax
B-cell lymphoma protein 2-associated X protein
- Bcl-2
B-cell lymphoma protein 2
- BET
Betaine
- BHMT
Betaine-homocysteine methyltransferase
- BiP
Binding immunoglobulin protein
- CDAHFD
choline-deficient, L-amino acid-defined, high-fat diet
- CDO
Cysteine dioxygenase
- CHOP
C/EBP homologous protein
- CQ
Chloroquine
- CβS
Cystathionine β-synthase
- CγL
Cystathionine γ-lyase
- DNL
De novo lipogenesis
- ER
Endoplasmic reticulum
- ERK
Extracellular signal-regulated kinase
- FAO
Fatty acid oxidation
- FFA
Free fatty acids
- GAPDH
Glyceraldehyde-3-phosphate dehydrogenase
- GCLC
Glutamate-cysteine ligase catalytic subunit
- GSH
Glutathione
- H&E
Hematoxylin and eosin
- HCY
Homocysteine
- ICR
Institute of Cancer Research
- IP
Intraperitoneal
- IR
Insulin resistance
- JNK
c-Jun N-terminal kinase
- LC3
Microtubule-associated protein light chain 3
- MAFLD
Metabolic dysfunction-associated fatty liver disease
- MAT
Methionine adenosyltransferase
- MCD
Methionine/choline-deficient
- mTOR
Mammalian target of rapamycin
- NAFLD
Non-alcoholic fatty liver disease
- NASH
Non-alcoholic steatohepatitis
- p62/SQSTM1
ubiquitin-binding protein p62/Sequestrosome-1
- PARP
Poly (ADP-ribose) polymerase
- PC
Phosphatidylcholine
- PE
Phosphatidylethanolamine
- PEMT
Phosphatidylethanolamine methyltransferase
- PPARα
peroxisome proliferator-activated receptor alpha
- SAA
S-amino acid
- SAH
S-adenosylhomocystiene
- SAHH
S-adenosylhomocysteine hydroxylase
- SAM
S-adenosylmethioine
- SBD-F
7-fluorobenzofuranzan-4-sulfonic acid
- TBARS
Thiobarbituric acid-reactive substances
- TG
Triglyceride
- VLDL
Very-low density lipoprotein
1. Introduction
Non-alcoholic fatty liver disease (NAFLD) is a pathophysiological condition characterized by hepatic lipid accumulation that is not caused by excessive alcohol consumption, and about 25% of adult population is known to have NAFLD (Ipsen et al., 2018; Bessone et al., 2019; Iqbal et al., 2019). Metabolic syndromes such as obesity, insulin resistance (IR), high blood pressure, and hyperlipidemia are the risk factors for NAFLD, and thus the term ‘metabolic dysfunction-associated fatty liver disease (MAFLD)’ has been suggested from 2020 for the diagnosis of this disease (Ipsen et al., 2018; Bessone et al., 2019; Iqbal et al., 2019; Gofton et al., 2023). Hepatic fat accumulation in NAFLD results from the dysregulation of lipid metabolism in the liver, including increased lipid uptake and de novo lipogenesis (DNL) and decreased fatty acid oxidation (FAO) and lipid export (Ipsen et al., 2018; Bessone et al., 2019). Recently, downregulation of lipid degradation by autophagy, called lipophagy, has been suggested as a mechanism of steatosis (Khambu et al., 2018; Bessone et al., 2019; Grefhorst et al., 2021). NAFLD progresses from simple steatosis to non-alcoholic steatohepatitis (NASH) with inflammation, fibrosis, cirrhosis, and hepatocellular carcinoma (Bessone et al., 2019; Iqbal et al., 2019). In NAFLD, hepatocytes are damaged via several mechanisms such as oxidative stress, lipotoxicity, inflammation, endoplasmic reticulum (ER) stress, and apoptosis (Bessone et al., 2019; Iqbal et al., 2019). Although NAFLD is considered a public health problem, there are no FDA-approved drugs for its treatment.
Autophagy is a cellular degradation process that involves the formation of autolysosomes that degrade organelles, proteins, and lipids to produce ATP when cellular energy is low (Khambu et al., 2018; Bessone et al., 2019; Grefhorst et al., 2021). The proper regulation of autophagy is beneficial because it removes not only lipid droplets but also damaged mitochondria and unfolded/misfolded proteins (Khambu et al., 2018; Bessone et al., 2019; Grefhorst et al., 2021). Downregulation of autophagy in chronic NAFLD can cause lipid accumulation, mitochondrial oxidative stress, ER stress, and apoptosis in the liver (Khambu et al., 2018; Bessone et al., 2019; Grefhorst et al., 2021). Thus, activation of autophagy has been suggested as a therapeutic strategy for NAFLD.
S-amino acid (SAA) metabolism, mainly found in the mammalian liver, is a metabolic pathway involving methionine and cysteine (Kwon et al., 2009; Jung, 2015). Methionine is converted to S-adenosylmethionine (SAM) by methionine adenosyltransferase (MAT), which donates a methyl group for various methylation reactions. The demethylated product S-adenosylhomocysteine (SAH) is hydrolyzed to homocysteine (HCY), which then undergoes remethylation or transsulfuration. HCY is remethylated to methionine by betaine-homocysteine methyltransferase (BHMT) or methionine synthase. In contrast, serial reactions catalyzed by cystathionine β-synthase (CβS) and cystathionine-lyase (CγL) convert HCY to cysteine. Cysteine is used to synthesize glutathione (GSH) and taurine via the rate-limiting enzymes glutamate-cysteine ligase (GCL) and cysteine dioxygenase (CDO), respectively. The dysregulation of SAA metabolism is closely associated with various liver diseases, including NAFLD (Kwon et al., 2009; Wang et al., 2014; Jung, 2015). A reduced SAM/SAH ratio, elevated homocysteine levels, and decreased GSH levels are thought to cause hepatic steatosis, ER stress, and oxidative stress in NAFLD (Kwon et al., 2009; Wang et al., 2014; Jung, 2015; Werge et al., 2021).
Betaine is a phytochemical found in goji berry, sugar beets, spinach, wheat germ, and shrimp (Arumugam et al., 2021; Wang et al., 2021). In the human body, betaine acts as an osmoregulator and methyl donor for BHMT-mediated HCY remethylation. Betaine acts as a hepatoprotectant against alcoholic liver disease and NAFLD (Arumugam et al., 2021; Wang et al., 2021). Betaine supplementation alleviates the liver injury, steatosis, inflammation, and fibrosis induced by high-fat, high-sugar, and methionine/choline-deficient (MCD) diets (Song et al., 2007; Kwon et al., 2009; Kathirvel et al., 2010; Wang et al., 2014; Deminice et al., 2015; Xu et al., 2015; Ahn et al., 2016; Ge et al., 2016; Vesković et al., 2019; Vesković et al., 2020). The proposed mechanisms of hepatoprotective and anti-steatogenic actions include improvements in hepatic antioxidant capacity, lipid export, DNL, and FAO, which are associated with the restoration of the hepatic concentrations of SAA metabolites, such as the SAM, HCY and GSH (Kwon et al., 2009; Kathirvel et al., 2010; Deminice et al., 2015; Ahn et al., 2016). Recently, betaine was reported to upregulate autophagy but inhibit ER stress and apoptosis in NAFLD models (Ge et al., 2016; Vesković et al., 2019, 2020). However, the exact relationship between the regulation of SAA metabolism and autophagy activation by betaine remains unclear. Moreover, the role of autophagy in betaine-mediated suppression of cellular stress is elusive.
This study aimed to elucidate the mechanisms underlying the hepatoprotective action of betaine against NAFLD by examining hepatic SAA metabolism, steatosis, ER stress, and apoptosis. Specifically, the contribution of autophagy to the betaine-promoted improvement of NAFLD was investigated.
2. Materials and methods
2.1. Animal treatments
Male Institute of Cancer Research (ICR) mice (7-week-old, n = 40, Hyochang Science, Daegu, Republic of Korea) were housed in mouse cages in the animal facility of Pusan National University under controlled conditions (22 ± 2 °C, 55 ± 5% humidity, and a 12 h light-dark cycle), and were acclimated for 1 week before the experiments. Animal protocols were approved by the Institutional Animal Care and Use Committee of Pusan National University (PNU-2021-0051). The first experiment was designed to evaluate the protective effects of betaine against NAFLD induced by a choline-deficient L-amino acid-defined high-fat diet (CDAHFD). Mice (n = 20) were randomly divided into four groups. Control (n = 5) mice were fed normal rodent chow and filtered tap water. The CDAHFD group (n = 5) was fed a commercial rodent diet with high-fat content (60% of total calories) and 0.1% methionine without choline (A06071302; Research Diets, Inc., New Brunswick, NJ, USA) for 1 week to induce NAFLD. Betaine (B2629, Merck KGaA, Darmstadt, Germany) at 0.2% (n = 5) or 1% (n = 5) in drinking water was administered to the mice fed with CDAHFD for 1 week. The second experiment was conducted to identify the hepatoprotective mechanism of betaine against NAFLD associated with autophagy. Mice were fed normal chow (control; n = 5), CDAHFD (n = 5), or CDAHFD with betaine (0.5%) in drinking water (n = 5) for 1 week. The last group (n = 5) was intraperitoneally injected with chloroquine (20 mg/kg, C6628, Merck KGaA) once a day to inhibit autophagy during the 1-week supplementation of CDAHFD with betaine (0.5% in drinking water). After the experiments, serum and liver samples were obtained and stored at −80 °C.
2.2. Histopathological examination
Liver tissues were fixed in 10% buffered formalin, and the sectioned tissues were subjected to hematoxylin and eosin (H&E) and oil-red O staining for observation of hepatic lipid droplets.
2.3. Determination of hepatic injury and oxidative stress
Serum alanine transaminase (ALT) activity was determined to examine liver injury using an ALT assay kit (Asan Pharm, Seoul, Republic of Korea). To quantify thiobarbituric acid-reactive substances (TBARS), such as malondialdehyde, liver homogenates were allowed to react with trichloroacetic acid (5%), TBA (0.25%), and HCl (0.17 M) for 40 min at 90 °C. The absorbance of the supernatant was measured at 535 nm.
2.4. Blood and hepatic lipid contents
Liver tissue was homogenized in a buffer (0.154 M KCl, 50 mM Tris-HCl, and 1 mM EDTA, pH 7.4), and lipids were extracted using chloroform and methanol. After centrifugation at 10,000 g for 5 min, the triglyceride (TG) content in the chloroform phase and serum was determined using a TG assay kit (Asan Pharm). Hepatic free fatty acids (FFA) were quantified using a commercial kit (Biomax Co., Ltd., Seoul, Korea).
2.5. Sulfur-containing amino acids and related metabolites
Hepatic SAA and its metabolites were quantified according to previously reported protocols (Kwon et al., 2020). Methionine and taurine were quantified by HPLC using a fluorescence detector (ex 338 nm and em 425 nm; Thermo Fisher Scientific, Waltham, MA, USA). The liver homogenate was mixed with methanol, and the centrifuged supernatant was used for the O-phthalaldehyde-derivatization of the molecules. The sample was separated by the Hector T-C18 column (3 μm × 4.6 mm x 100 mm; RStech, Daejeon, Republic of Korea) with mobile phase A (0.1 M sodium acetate, pH 7.2) and B (methanol and tetrahydrofuran, 97:3) at a flow rate of 1 mL/min at 35 °C. For SAM and SAH detection, the liver homogenate was mixed with perchloric acid (6%), and the centrifuged mixture was separated by the Kromasil 100-5-C18 column (5 μm × 4.6 mm × 250 mm; Kromasil, Bohus, Sweden) with mobile phases (6% acetonitrile, 8 mM 1-heptane sulfonate, and 40 mM ammonium phosphate buffer; pH 5; flow rate 1 mL/min) at 35 °C. The separated SAM and SAH were detected at 254 nm using a UV detector (Thermo Fisher Scientific). Homocysteine, cysteine, and GSH in the perchloric acid-treated liver homogenates were quantified after derivatization with 7-fluorobenzofuranzan-4-sulfonic acid (SBD-F, 0.025%). Samples were separated using an HPLC-connected reversed phase column (3 μm × 4.6 mm x 75 mm; RStech Corp, Cheongju, Korea) with the following mobile phases: 0.1 M sodium acetate (pH 4.5) and methanol (97:3). The metabolites were detected using the fluorescence detector (excitation 385 nm and emission 515 nm).
2.6. Immunoblotting
The liver homogenate was lysed using CETi Lysis Buffer (TransLab Biosciences, Daejeon, Korea), and the protein content of the centrifuged sample was determined using a bicinchoninic acid assay kit (BioVision Inc., Milpitas, CA, USA). Equal amounts of proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred onto a nitrocellulose membrane (Bio-Rad Laboratories, Inc., Hercules, CA, USA). Proteins were then reacted with primary and secondary antibodies, and the blots were visualized using an EZ-Western Lumi Pico detection kit (DoGenBio, Seoul, Korea). The primary antibodies against BHMT, MAT1A, SAHH, CβS, CγL, CDO, ERK, p-ERK, JNK, p-JNK, Bax, Bcl2, and GAPDH were purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA). PEMT antibody was purchased from MyBioSource, Inc. (San Diego, CA, USA). GCLC and PPARα antibodies were purchased from Abcam (Cambridge, UK). The primary antibodies against AMPK, pThr172-AMPK, p-ACC, p62/SQSTM1, ATG7, LC3B, mTOR, pSer2448-mTOR, BiP, CHOP, caspase-3, cleaved caspase-3, PARP, cleaved PARP, and ACS1 were purchased from Cell Signaling Technology, Inc. (Danvers, MA, USA). ATF6 antibody was purchased from Novus Biologicals (Englewood, CO, USA).
2.7. Statistical analysis
The results are expressed as mean ± standard deviation (SD), and the statistical differences were analyzed by one-way analysis of variance (ANOVA) followed by Newman-Keuls multiple comparison test using GraphPad Prism (v5.0; GraphPad Software Inc.; La Jolla, CA, USA). The level of significance was set at p < 0.05.
3. Results and discussion
3.1. Alleviation of CDAHFD-induced liver injury and steatosis by betaine
Rodent diet-feeding models have been developed for NAFLD research using high-fat, high-cholesterol, high-sugar, and MCD diets (Santhekadur et al., 2018). The most commonly used high-fat mouse model shows obesity, IR, and steatosis, similar to those observed in NAFLD patients (Santhekadur et al., 2018). However, hepatic cell death, inflammation, and fibrosis were hardly induced even after a 16-week feeding (Santhekadur et al., 2018). In contrast, the MCD diet induces severe oxidative liver damage, inflammation, fibrosis, and fatty liver after only 1- or 2-week supplementation (Santhekadur et al., 2018). This model causes significant weight loss without IR, which differs from that in NAFLD patients (Santhekadur et al., 2018). The recently developed CDAHFD (high-fat, 60 kcal%; low-methionine, 0.1%; without choline) model has several advantages, such as the rapid induction of NASH with oxidative stress, inflammation, and fibrosis after 1-week of feeding without weight loss (Matsumoto et al., 2013; Lee et al., 2019; Sugasawa et al., 2021). Therefore, we used a CDAHFD model to identify the protective mechanism of betaine against NAFLD. CDAHFD feeding for 1 week caused significant hepatotoxicity and steatosis in mice, as indicated by the elevated serum ALT activity, liver/body weight ratio, and hepatic TG levels (Fig. 1). However, betaine supplementation significantly prevented these CDAHFD-induced adverse effects in a dose-dependent manner (Fig. 1). The CDAHFD-induced decrease in serum TG level was reversed by betaine, suggesting that betaine affects lipid transport between the liver and blood, probably via alteration of SAA metabolism. The CDAHFD-induced increase in hepatic TBARS level, an oxidative stress marker, was normalized by betaine, indicating its anti-oxidative effect (Fig. 1).
Fig. 1.
Effects of betaine on liver injury, steatosis, and oxidative stress in choline-deficient, L-amino acid-defined, high-fat diet (CDAHFD)-induced non-alcoholic fatty liver disease (NAFLD) in mice. Male mice (n = 5 in each group) were fed CDAHFD with or without betaine (BET, 0.2 or 1 %) in drinking water for 1 week. Results are presented as mean ± SD. The letters (a, b, and c) indicate the comparison among the groups; the values with different letters are statistically different (one-way ANOVA followed by Newman-Keuls multiple comparison test). CV, central vein.
3.2. Effects of betaine on CDAHFD-induced dysregulation of hepatic SAA metabolism
Changes in hepatic SAA metabolism, as characterized by decreased SAM and GSH levels and increased HCY level in the liver, reportedly occur in NAFLD induced by high-fat diet and MCD (Kwon et al., 2009; Caballero et al., 2010; Wang et al., 2014). This metabolic pathway is considered important in NAFLD, because the restoration of metabolites alleviates the severity of NAFLD (Kwon et al., 2009; Wang et al., 2014). In this study, hepatic methionine, SAM, SAM/SAH ratio, and cysteine concentrations were decreased by the CDAHFD, whereas homocysteine and taurine levels were increased (Fig. 2). This is similar to the results of previous animal NAFLD studies using high-fat and MCD diets (Kwon et al., 2009; Caballero et al., 2010; Wang et al., 2014). However, betaine supplementation reversed all the changes promoted by CDAHFD, as characterized by the increase in BHMT, MAT1A, CβS, and CγL and decrease in CDO proteins (Fig. 2.). The SAM/SAH ratio, a cellular methylation potential parameter, affects hepatic lipid export via very-low-density lipoprotein (VLDL) production (Cole et al., 2012; Bessone et al., 2019). PEMT, which synthesizes phosphatidylcholine from phosphatidylethanolamine, requires a methyl group in SAM (Cole et al., 2012; Bessone et al., 2019). As phosphatidylcholine is an abundant component of VLDL, the SAM/SAH ratio is important for hepatic VLDL production (Cole et al., 2012; Bessone et al., 2019). Previous studies have reported that betaine ameliorates steatosis by increasing VLDL secretion, which is impaired in NAFLD, as shown by the increased hepatic expression of microsomal triglyceride transfer protein and apolipoprotein B100 for VLDL synthesis (Sparks et al., 2006; Wang et al., 2014; Ge et al., 2016; Bessone et al., 2019). Thus, the betaine-induced normalization of SAM/SAH and increase in PEMT protein (Fig. 2) may be the mechanism underlying the reduction in hepatic lipid content via lipid secretion (Fig. 1).
Fig. 2.
Effects of betaine on choline-deficient, L-amino acid-defined, high-fat diet (CDAHFD)-induced dysregulation of S-amino acid metabolism in mice. Male mice (n = 5 in each group) were fed CDAHFD with or without betaine (BET, 0.2 or 1 %) in drinking water for 1 week. Results are presented as mean ± SD. The letters (a, b, c, and d) indicate the comparison among the groups; the values with different letters are statistically different (one-way ANOVA followed by Newman-Keuls multiple comparison test).
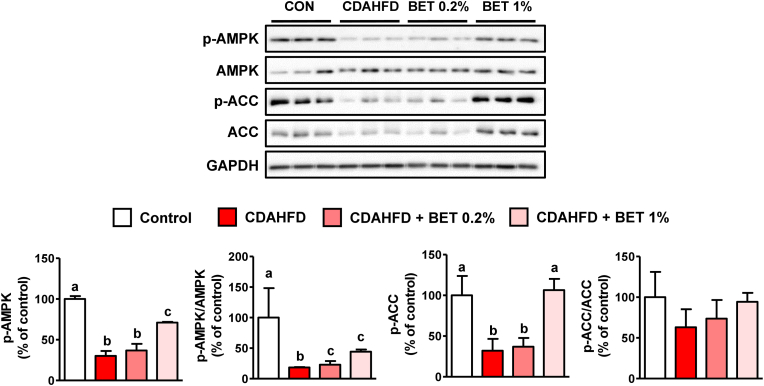
3.3. Effects of betaine on CDAHFD-induced changes in AMPK and ACC phosphorylation
AMPK is a regulatory protein involved in lipid metabolism, including DNL and FAO (Bessone et al., 2019). Activated AMPK inhibits the ACC-mediated formation of malonyl-CoA, which is used for fatty acid synthesis (Bessone et al., 2019). Malonyl-CoA is also an inhibitor of carnitine palmitoyltransferase-1, which transfers fatty acids to the mitochondria (Bessone et al., 2019). Thus, AMPK activation leads to the downregulation of fatty acid synthesis and stimulation of mitochondrial FAO (Bessone et al., 2019) and has been proposed as a mechanism for betaine-mediated improvement of steatosis in NAFLD induced by a high-fat or high-sucrose diet (Song et al., 2007; Kathirvel et al., 2010; Xu et al., 2015). In our study, phosphorylated AMPK (p-AMPK, activated form of AMPK) and phosphorylated ACC (p-ACC, inhibited form of ACC) were both significantly diminished by CDAHFD but were increased by betaine (Fig. 3). This suggests that betaine improves hepatic steatosis via AMPK-mediated regulation of lipid metabolism. Hepatic HCY is reportedly an important modulator of AMPK activity. In H4IIE liver cells, HCY elevation by CβS inhibition or HCY treatment decreased p-AMPK levels; however, betaine reversed AMPK activity via BHMT-mediated HCY remethylation (Ahn et al., 2016). Moreover, normalization of the increased HCY concentration by betaine in MCD-fed mouse livers resulted in improved AMPK activity and steatosis (Ahn et al., 2016). SAM is known to be an activator of CβS (Finkelstein et al., 1975; Prudova et al., 2006); thus, the BHMT- and CβS-mediated reduction of HCY by betaine can be the reason of AMPK activation in CDAHFD-fed mice liver.
Fig. 3.
Effects of betaine on AMPK and ACC protein levels in choline-deficient, L-amino acid-defined, high-fat diet (CDAHFD)-fed mouse livers. Male mice (n = 5 in each group) were fed CDAHFD with or without betaine (BET, 0.2 or 1 %) in drinking water for 1 week. Results are presented as mean ± SD. The letters (a, b, and c) indicate the comparison among the groups; the values with different letters are statistically different (one-way ANOVA followed by Newman-Keuls multiple comparison test).
3.4. Attenuation of CDAHFD-induced ER stress and apoptosis by betaine
ER stress is a cellular condition in which unfolded and misfolded proteins accumulate in the ER (Malhotra and Kaufman, 2011). Various stress signals such as oxidative stress disturb proper protein folding in this organelle, resulting in the activation of the unfolded protein response (UPR) (Malhotra and Kaufman, 2011). UPR inhibits protein synthesis, induces chaperones, and activates protein degradation systems to reduce abnormal protein accumulation (Malhotra and Kaufman, 2011). However, severe and prolonged ER stress promotes calcium release and mitochondrial-mediated apoptosis (Malhotra and Kaufman, 2011). Thus, ER stress has been suggested as a mechanism underlying liver injury in NAFLD (Han and Kaufman, 2016; Song and Malhi, 2019). In this study, CDAHFD increased ER stress markers, bound immunoglobulin protein (BiP), and activated transcription factor 6 (ATF6), but betaine reversed these effects (Fig. 4). C/EBP homologous protein (CHOP), which is associated with the induction of apoptosis under ER stress (Sano and Reed, 2013; Iurlaro and Muñoz-Pinedo, 2016) was also increased by CDAHFD but normalized by betaine (Fig. 4). Mitogen-activated protein kinases (MAPKs) such as extracellular signal-regulated kinase (ERK) and c-Jun N-terminal kinase (JNK) are activated by ER stress (Sano and Reed, 2013; Iurlaro and Muñoz-Pinedo, 2016); thus, the decrease in phosphorylated ERK and phosphorylated JNK which was induced by CDAHFD also indicates the alleviation of ER stress by betaine (Fig. 4.). Betaine also inhibited the CDAHFD-induced hepatic apoptosis promoted by CDAHFD (Fig. 4). Apoptotic B-cell lymphoma protein 2-associated X protein (Bax) increased, but anti-apoptotic B-cell lymphoma protein 2 (Bcl-2) decreased in the livers of CDAHFD-fed mice; however, betaine normalized those Bcl2 family proteins. Caspase-3, an executive protease involved in apoptosis, cleaves poly (ADP-ribose) polymerase (PARP), thereby preserving ATP during apoptosis (Boulares et al., 1999; Sano and Reed, 2013; Iurlaro and Muñoz-Pinedo, 2016). Thus, betaine promoted a decrease in the cleaved forms of caspase-3 and PARP, which were elevated by CDAHFD, suggesting an anti-apoptotic action of betaine in NAFLD. ER stress can cause apoptosis; thus, the inhibition of ER stress by betaine can prevent apoptosis. The reduction of HCY by betaine could also contribute to the attenuation of liver injury because HCY has been reported to induce ER stress (Ai et al., 2017; Shen et al., 2022). Excessive fatty acid levels cause ER stress, which negatively affects lipid metabolism and VLDL synthesis (Han and Kaufman, 2016; Song and Malhi, 2019). Therefore, the betaine-promoted reduction in ER stress and steatosis appears to be linked to the alleviation of CDAHFD-induced NAFLD.
Fig. 4.
Effects of betaine on the endoplasmic reticulum stress and apoptosis induced by a choline-deficient, L-amino acid-defined, high-fat diet (CDAHFD) in mouse livers. Male mice (n = 5 in each group) were fed CDAHFD with or without betaine (BET, 0.2 or 1 %) in drinking water for 1 week. Results are presented as mean ± SD. The letters (a, b, and c) indicate the comparison among the groups, and the values with different letters are statistically different (one-way ANOVA followed by Newman-Keuls multiple comparison test).
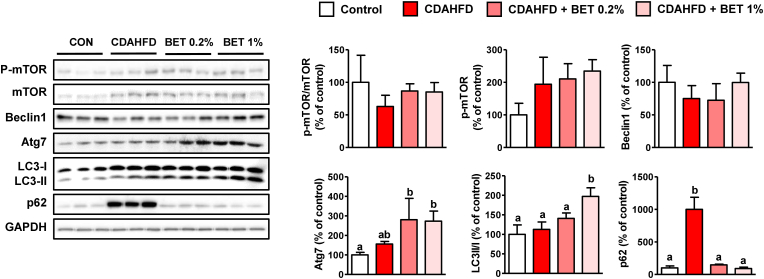
3.5. Activation of CDAHFD-suppressed autophagy by betaine
Autophagy is known to play a role not only in cellular component degradation but also in energy metabolism (Martinez-Lopez and Singh, 2015; Mao et al., 2016). Autophagy can alleviate ER stress and steatosis by degrading misfolded proteins and lipid droplets, respectively (Martinez-Lopez and Singh, 2015; Mao et al., 2016). Downregulation of autophagy observed in chronic NAFLD suggests that this degradation pathway may be a therapeutic target for NAFLD (Martinez-Lopez and Singh, 2015; Mao et al., 2016). AMPK directly activates autophagy and inhibits mammalian target of rapamycin (mTOR) which suppresses initiation of autophagy (Martinez-Lopez and Singh, 2015; Mao et al., 2016). Beclin1 is a protein involved in the formation of isolated phagophore membranes, and autophagy-related 7 (Atg7) acts as an E1 ligase that binds microtubule-associated protein light chain 3 (LC3) to PE (Mao et al., 2016). Lipid-conjugated LC3-II, located on the phagophore membrane, recruits proteins, such as ubiquitin-binding protein p62 (p62), a cargo receptor for ubiquitinated macromolecules (Martinez-Lopez and Singh, 2015). Organelles, proteins, and lipids engulfed by autophagosomes are degraded by lysosomal acidic hydrolases after their fusion with lysosomes (Martinez-Lopez and Singh, 2015; Mao et al., 2016). Our results showed that betaine increased the Atg7 and LC3II/I ratios, indicating the activation of autophagic flux (Fig. 5). P62, which accumulates when autophagy is inhibited, was significantly increased by the CDAHFD but normalized by betaine (Fig. 5). These results suggested that betaine stimulated autophagy, which was suppressed by CDAHFD. Because betaine increased p-AMPK, the induction of autophagy by this protein appears to be a possible mechanism for the betaine-mediated attenuation of ER stress.
Fig. 5.
Effects of betaine on autophagy in choline-deficient, L-amino acid-defined, high-fat diet (CDAHFD)-fed mouse livers. Male mice (n = 5 in each group) were fed a CDAHFD with or without betaine (BET, 0.2 or 1 %) in drinking water for 1 week. Results are presented as mean ± SD. The letters (a and b) indicate the comparison among the groups; the values with different letters are statistically different (one-way ANOVA followed by Newman-Keuls multiple comparison test).
3.6. Effects of chloroquine on betaine-promoted hepatoprotection and autophagy in CDAHFD-fed mice liver
CQ is a lysosomotropic agent used for the prevention and treatment of malaria. CQ has been used in autophagy research because it inhibits autophagic flux by decreasing the fusion of autophagosomes and lysosomes, as well as lysosomal acidification (Mauthe et al., 2018). CQ treatment in NAFLD models such as high-fat and MCD diets aggravates steatosis by impairing autophagy (Chen et al., 2016; Zhang et al., 2018). Previous studies have shown that betaine increased autophagy in MCD diet-fed mice, as shown by the elevations of Atg 4 and 5, Beclin1 mRNA levels, LC3II/I ratio, and p-mTOR, but resulted in a decrease in p62 protein (Vesković et al., 2019, 2020). However, the contribution of autophagy activation to NAFLD prevention in betaine-treated livers remained uncertain. Thus, we injected CQ into mice fed with CDAHFD and betaine to identify the role of autophagy. CQ treatment partially reversed the betaine-mediated decrease in liver weight, serum ALT activity, and hepatic TG concentration without altering FFA levels (Fig. 6). These hepatic deteriorations induced by CQ appear to be due to the inhibition of autophagic degradation, as shown in Fig. 7. The increased Atg7 and LC3II/I ratios induced by betaine were not affected by CQ (Fig. 7). However, CQ increased p62 protein levels compared to the CDAHFD + betaine group (Fig. 7). Thus, it appears that CQ did not affect autophagy initiation by betaine but inhibited autolysosomal degradation. The fact that CQ did not completely suppress autophagy may explain its partial aggravation of steatosis and hepatotoxicity. Nevertheless, these results indicate the possible role of autophagy as an upstream signal for betaine-mediated improvement in NAFLD.
Fig. 6.
Effects of chloroquine (CQ) on betaine-mediated non-alcoholic fatty liver disease (NAFLD) improvement in choline-deficient, L-amino acid-defined, high-fat diet (CDAHFD)-fed mice. CQ (20 mg/kg) was daily injected (IP) into male mice (n = 5 in each group) fed with CDAHFD and betaine (BET, 0.5 % in drinking water) for 1 week. Results are presented as mean ± SD. The letters (a, b, c, and d) indicate the comparison among the groups; the values with different letters are statistically different (one-way ANOVA followed by Newman-Keuls multiple comparison test).
Fig. 7.
Effects of chloroquine (CQ) on hepatic autophagy in mice fed a choline-deficient, L-amino acid-defined, high-fat diet (CDAHFD) and betaine. CQ (20 mg/kg) was daily injected (IP) into male mice (n = 5 in each group) fed with CDAHFD and betaine (BET, 0.5 % in drinking water) for 1 week. Results are presented as mean ± SD. The letters (a, b, and c) indicate the comparison among the groups; the values with different letters are statistically different (one-way ANOVA followed by Newman-Keuls multiple comparison test).
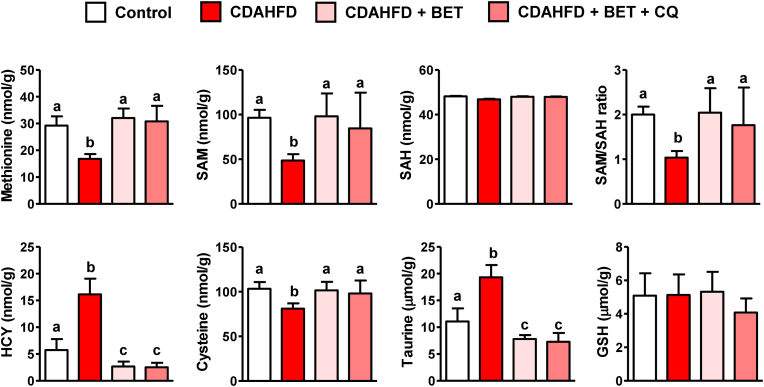
3.7. Effects of chloroquine on hepatic SAA metabolism
To investigate the association between the regulation of SAA metabolism and the induction of autophagy by betaine, SAA metabolites were analyzed in the livers of CDAHFD-fed mice treated with betaine only or betaine + CQ. Inhibition of autophagy did not affect the betaine-mediated normalization of SAA metabolism (Fig. 8), suggesting that hepatic SAA metabolism was not modulated by autophagy. Rather, these results suggest that SAA metabolism regulates autophagy, probably via HCY-mediated AMPK signaling.
Fig. 8.
Effects of chloroquine (CQ) on hepatic S-amino acid metabolism in mice fed a choline-deficient, L-amino acid-defined, high-fat diet (CDAHFD) and betaine. CQ (20 mg/kg) was daily injected (IP) into male mice (n = 5 in each group) fed with CDAHFD and betaine (BET, 0.5 % in drinking water) for 1 week. Results are presented as mean ± SD. The letters (a, b, and c) indicate the comparison among the groups; the values with different letters are statistically different (one-way ANOVA followed by Newman-Keuls multiple comparison test).
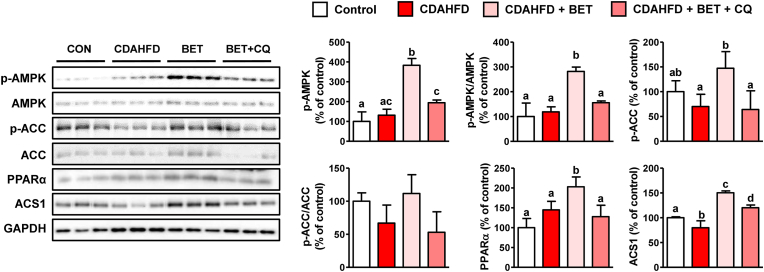
3.8. Effects of chloroquine on lipid metabolism
To identify the reason for the CQ-induced exacerbation of steatosis, AMPK and ACC protein levels were determined. Interestingly, betaine treatment decreased the p-AMPK and p-ACC protein levels (Fig. 9). This indicates that AMPK, an inducer of autophagy, can also be inhibited by autophagy suppression, suggesting aggravation of FAO and DNL. A transcription factor, peroxisome proliferator-activated receptor alpha (PPARα), and the target protein involved in FAO, acyl-CoA synthetase 1 (ACS1), were increased by betaine but decreased by CQ. These results support the hypothesis that autophagy is a mechanism underlying the restoration of lipid metabolism by betaine.
Fig. 9.
Effects of chloroquine (CQ) on hepatic lipid metabolism in mice fed a choline-deficient, L-amino acid-defined, high-fat diet (CDAHFD) and betaine. CQ (20 mg/kg) was daily injected (IP) into male mice (n = 5 in each group) fed with CDAHFD and betaine (BET, 0.5 % in drinking water) for 1 week. Results are presented as mean ± SD. The letters (a, b, c, and d) indicate the comparison among the groups; the values with different letters are statistically different (one-way ANOVA followed by Newman-Keuls multiple comparison test).
3.9. Effects of chloroquine on ER stress and apoptosis signaling
Autophagy is closely linked to ER stress because it removes unfolded/misfolded proteins from the ER (Mao et al., 2016) as mentioned above. Therefore, inhibition of autophagy can lead to ER stress, which in turn promotes apoptosis (Mao et al., 2016). In our study, the ER stress markers BiP, ATF6, and CHOP, which were decreased by betaine in CDAHFD-fed mice livers, were increased by CQ (Fig. 10), indicating that betaine stimulation of autophagy is directly linked to reduced ER stress. Partial reversal of p-ERK and Bcl-2 proteins by CQ also suggested a role for autophagy in the amelioration of ER stress and apoptosis in betaine-treated mice with NAFLD (Fig. 10).
Fig. 10.
Effects of chloroquine (CQ) on hepatic ER stress and apoptosis in mice fed with choline-deficient, L-amino acid-defined, high-fat diet (CDAHFD) and betaine. CQ (20 mg/kg) was daily injected (IP) into male mice (n = 5 in each group) fed with CDAHFD and betaine (BET, 0.5 % in drinking water) for 1 week. Results are presented as mean ± SD. The letters (a, b, c, d) indicate the comparison among the groups; the values with different letters are statically different from one another (one-way ANOVA followed by Newman-Keuls multiple comparison test).
4. Conclusions
In this study, we showed, for the first time, that betaine protects the liver from CDAHFD-induced NAFLD. The stimulation of HCY remethylation and transsulfuration, resulting in an increased SAM/SAH ratio and decreased HCY levels, appears to be linked to the amelioration of steatosis, ER stress, and apoptosis. Moreover, activation of AMPK and autophagy by betaine may be important mechanisms for improving NAFLD. Specifically, we demonstrated that betaine-stimulated autophagy can act as an upstream signal for reduced liver injury, steatosis, ER stress, and apoptosis in CDAHFD-fed mouse livers using an autophagy inhibitor, CQ. Although the direct association between the regulation of SAA metabolism and autophagy remains unclear, our results suggest that autophagy plays an important role in betaine-promoted hepatoprotection against NAFLD.
CRediT authorship contribution statement
Jinuk Seo: Formal analysis, Validation, Visualization, Writing – original draft. Doyoung Kwon: Conceptualization, Formal analysis, Validation, Visualization, Writing – original draft. Sou Hyun Kim: Formal analysis, Visualization. Mi Ran Byun: Writing – review & editing. Yun-Hee Lee: Conceptualization, Supervision, Writing – review & editing. Young-Suk Jung: Conceptualization, Supervision, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This study was supported by the National Research Foundation of Korea (NRF) funded by the Korea government (MSIT) [NRF-2019R1I1A3A01058584]. This research was also supported by Basic Science Research Program to Research Institute for Basic Sciences (RIBS) of Jeju National University through the NRF funded by the Ministry of Education [2019R1A6A1A10072987].
Handling Editor: Dr. Quancai Sun
Contributor Information
Yun-Hee Lee, Email: yunhee.lee@snu.ac.kr.
Young-Suk Jung, Email: youngjung@pusan.ac.kr.
Data availability
Data will be made available on request.
References
- Ahn C.W., Jun D.S., Na J.D., Choi Y.J., Kim Y.C. Alleviation of hepatic fat accumulation by betaine involves reduction of homocysteine via up-regulation of betaine-homocysteine methyltransferase (BHMT) Biochem. Biophys. Res. Commun. 2016;477:440–447. doi: 10.1016/j.bbrc.2016.06.080. [DOI] [PubMed] [Google Scholar]
- Ai Y., Sun Z., Peng C., Liu L., Xiao X., Li J. Homocysteine induces hepatic steatosis involving ER stress response in high methionine diet-fed mice. Nutrients. 2017;9:346. doi: 10.3390/nu9040346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arumugam M.K., Paal M.C., Donohue T.M., Jr., Ganesan M., Osna N.A., Kharbanda K.K. Beneficial effects of betaine: a Comprehensive review. Biology. 2021;10:456. doi: 10.3390/biology10060456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessone F., Razori M.V., Roma M.G. Molecular pathways of nonalcoholic fatty liver disease development and progression. Cell. Mol. Life Sci. 2019;76:99–128. doi: 10.1007/s00018-018-2947-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulares A.H., Yakovlev A.G., Ivanova V., Stoica B.A., Wang G., Iyer S., Smulson M. Role of poly(ADP-ribose) polymerase (PARP) cleavage in apoptosis. Caspase 3-resistant PARP mutant increases rates of apoptosis in transfected cells. J. Biol. Chem. 1999;274:22932–22940. doi: 10.1074/jbc.274.33.22932. [DOI] [PubMed] [Google Scholar]
- Caballero F., Fernández A., Matías N., Martínez L., Fucho R., Elena M., Caballeria J., Morales A., Fernández-Checa J.C., García-Ruiz C. Specific contribution of methionine and choline in nutritional nonalcoholic steatohepatitis: impact on mitochondrial S-adenosyl-L-methionine and glutathione. J. Biol. Chem. 2010;285:18528–18536. doi: 10.1074/jbc.M109.099333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R., Wang Q., Song S., Liu F., He B., Gao X. Protective role of autophagy in methionine-choline deficient diet-induced advanced nonalcoholic steatohepatitis in mice. Eur. J. Pharmacol. 2016;770:126–133. doi: 10.1016/j.ejphar.2015.11.012. [DOI] [PubMed] [Google Scholar]
- Cole L.K., Vance J.E., Vance D.E. Phosphatidylcholine biosynthesis and lipoprotein metabolism. Biochim. Biophys. Acta. 2012;1821:754–761. doi: 10.1016/j.bbalip.2011.09.009. [DOI] [PubMed] [Google Scholar]
- Deminice R., da Silva R.P., Lamarre S.G., Kelly K.B., Jacobs R.L., Brosnan M.E., Brosnan J.T. Betaine supplementation prevents fatty liver induced by a high-fat diet: effects on one-carbon metabolism. Amino Acids. 2015;47:839–846. doi: 10.1007/s00726-014-1913-x. [DOI] [PubMed] [Google Scholar]
- Finkelstein J.D., Kyle W.E., Martin J.L., Pick A.M. Activation of cystathionine synthase by adenosylmethionine and adenosylethionine. Biochem. Biophys. Res. Commun. 1975;66:81–87. doi: 10.1016/s0006-291x(75)80297-x. [DOI] [PubMed] [Google Scholar]
- Ge C.X., Yu R., Xu M.X., Li P.Q., Fan C.Y., Li J.M., Kong L.D. Betaine prevented fructose-induced NAFLD by regulating LXRα/PPARα pathway and alleviating ER stress in rats. Eur. J. Pharmacol. 2016;770:154–164. doi: 10.1016/j.ejphar.2015.11.043. [DOI] [PubMed] [Google Scholar]
- Gofton C., Upendran Y., Zheng M.H., George J. MAFLD: How is it different from NAFLD? Clin. Mol. Hepatol. 2023;29:S17–S31. doi: 10.3350/cmh.2022.0367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grefhorst A., van de Peppel I.P., Larsen L.E., Jonker J.W., Holleboom A.G. The role of lipophagy in the development and treatment of non-alcoholic fatty liver disease. Front. Endocrinol. 2021;11 doi: 10.3389/fendo.2020.601627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J., Kaufman R.J. Role of ER stress in lipid metabolism and lipotoxicity. J. Lipid Res. 2016;57:1329–1338. doi: 10.1194/jlr.R067595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ipsen D.H., Lykkesfeldt J., Tveden-Nyborg P. Molecular mechanisms of hepatic lipid accumulation in non-alcoholic fatty liver disease. Cell. Mol. Life Sci. 2018;75:3313–3327. doi: 10.1007/s00018-018-2860-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iqbal U., Perumpail B.J., Akhtar D., Kim D., Ahmed A. The Epidemiology, risk Profiling and Diagnostic Challenges of nonalcoholic fatty liver disease. Medicine (Baltim.) 2019;6:41. doi: 10.3390/medicines6010041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iurlaro R., Muñoz-Pinedo C. Cell death induced by endoplasmic reticulum stress. FEBS J. 2016;283:2640–2652. doi: 10.1111/febs.13598. [DOI] [PubMed] [Google Scholar]
- Jung Y.S. Metabolism of sulfur-Containing amino acids in the liver: a link between hepatic injury and Recovery. Biol. Pharm. Bull. 2015;38:971–974. doi: 10.1248/bpb.b15-00244. [DOI] [PubMed] [Google Scholar]
- Kathirvel E., Morgan K., Nandgiri G., Sandoval B.C., Caudill M.A., Bottiglieri T., French S.W., Morgan T.R. Betaine improves nonalcoholic fatty liver and associated hepatic insulin resistance: a potential mechanism for hepatoprotection by betaine. Am. J. Physiol. Gastrointest. Liver Physiol. 2010;299:G1068–G1077. doi: 10.1152/ajpgi.00249.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khambu B., Yan S., Huda N., Liu G., Yin X.M. Autophagy in non-alcoholic fatty liver disease and alcoholic liver disease. Liver Res. 2018;2:112–119. doi: 10.1016/j.livres.2018.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon D., Jung Y.S., Kim S.J., Park H.K., Park J.H., Kim Y.C. Impaired sulfur-amino acid metabolism and oxidative stress in nonalcoholic fatty liver are alleviated by betaine supplementation in rats. J. Nutr. 2009;139:63–68. doi: 10.3945/jn.108.094771. [DOI] [PubMed] [Google Scholar]
- Kwon D., Kim S.H., Son S.W., Seo J., Jeong T.B., Kim K.M., Jung J.C., Jung M.S., Lee Y.H., Jung Y.S. Germinated soybean embryo extract ameliorates fatty liver injury in high-fat diet-fed obese mice. Pharmaceuticals. 2020;13:380. doi: 10.3390/ph13110380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S., Kwak J.H., Kim S.H., Jeong T.B., Son S.W., Kim J.H., Lim Y., Cho J.Y., Hwang D.Y., Kim K.S., Jung Y.S. Comparative study of liver injury induced by high-fat methionine- and choline-deficient diet in ICR mice originating from three different sources. Lab. Anim. Res. 2019;35:15. doi: 10.1186/s42826-019-0016-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra J.D., Kaufman R.J. ER stress and its functional link to mitochondria: role in cell survival and death. Cold Spring Harbor Perspect. Biol. 2011;3:a004424. doi: 10.1101/cshperspect.a004424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao Y., Yu F., Wang J., Guo C., Fan X. Autophagy: a new target for nonalcoholic fatty liver disease therapy. Hepat. Med. 2016;8:27–37. doi: 10.2147/HMER.S98120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Lopez N., Singh R. Autophagy and lipid droplets in the liver. Annu. Rev. Nutr. 2015;35:215–237. doi: 10.1146/annurev-nutr-071813-105336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto M., Hada N., Sakamaki Y., Uno A., Shiga T., Tanaka C., Ito T., Katsume A., Sudoh M. An improved mouse model that rapidly develops fibrosis in non-alcoholic steatohepatitis. Int. J. Exp. Pathol. 2013;94:93–103. doi: 10.1111/iep.12008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauthe M., Orhon I., Rocchi C., Zhou X., Luhr M., Hijlkema K.J., Coppes R.P., Engedal N., Mari M., Reggiori F. Chloroquine inhibits autophagic flux by decreasing autophagosome-lysosome fusion. Autophagy. 2018;14:1435–1455. doi: 10.1080/15548627.2018.1474314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prudova A., Bauman Z., Braun A., Vitvitsky V., Lu S.C., Banerjee R. S-adenosylmethionine stabilizes cystathionine beta-synthase and modulates redox capacity. Proc. Natl. Acad. Sci. USA. 2006;103:6489–6494. doi: 10.1073/pnas.0509531103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano R., Reed C.J. ER stress-induced cell death mechanisms. Biochim. Biophys. Acta. 2013;1833:3460–3470. doi: 10.1016/j.bbamcr.2013.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santhekadur P.K., Kumar D.P., Sanyal A.J. Preclinical models of non-alcoholic fatty liver disease. J. Hepatol. 2018;68:230–237. doi: 10.1016/j.jhep.2017.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen J., Jiao Y., Ding N., Xie L., Ma S., Zhang H., Yang A., Zhang H., Jiang Y. Homocysteine facilitates endoplasmic reticulum stress and apoptosis of hepatocytes by suppressing ERO1α expression via cooperation between DNMT1 and G9a. Cell Biol. Int. 2022;46:1236–1248. doi: 10.1002/cbin.11805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song M.J., Malhi H. The unfolded protein response and hepatic lipid metabolism in non-alcoholic fatty liver disease. Pharmacol. Ther. 2019;203 doi: 10.1016/j.pharmthera.2019.107401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Z., Deaciuc I., Zhou Z., Song M., Chen T., Hill D., McClain C.J. Involvement of AMP-activated protein kinase in beneficial effects of betaine on high-sucrose diet-induced hepatic steatosis. Am. J. Physiol. Gastrointest. Liver Physiol. 2007;293:G894–G902. doi: 10.1152/ajpgi.00133.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparks J.D., Collins H.L., Chirieac D.V., Cianci J., Jokinen J., Sowden M.P., Galloway C.A., Sparks C.E. Hepatic very-low-density lipoprotein and apolipoprotein B production are increased following in vivo induction of betaine-homocysteine S-methyltransferase. Biochem. J. 2006;395:363–371. doi: 10.1042/BJ20051966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugasawa T., Ono S., Yonamine M., Fujita S.I., Matsumoto Y., Aoki K., Nakano T., Tamai S., Yoshida Y., Kawakami Y., Takekoshi K. One week of CDAHFD induces steatohepatitis and mitochondrial dysfunction with oxidative stress in liver. Int. J. Mol. Sci. 2021;22:5851. doi: 10.3390/ijms22115851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vesković M., Labudović-Borović M., Mladenović D., Jadžić J., Jorgačević B., Vukićević D., Vučević D., Radosavljević T. Effect of betaine supplementation on liver tissue and ultrastructural changes in methionine-choline-deficient diet-induced NAFLD. Microsc. Microanal. 2020;26(2020):997–1006. doi: 10.1017/S1431927620024265. [DOI] [PubMed] [Google Scholar]
- Vesković M., Mladenovic D., Milenkovic M., Tosic J., Borozan S., Gopcevic K., Labudovic-Borovic M., Dragutinovic V., Vucevic D., Jorgacevic B., Isakovic A., Trajkovic V., Radosavljevic T. Betaine modulates oxidative stress, inflammation, apoptosis, autophagy, and Akt/mTOR signaling in methionine-choline deficiency-induced fatty liver disease. Eur. J. Pharmacol. 2019;848:39–48. doi: 10.1016/j.ejphar.2019.01.043. [DOI] [PubMed] [Google Scholar]
- Wang C., Ma C., Gong L., Dai S., Li Y. Preventive and therapeutic role of betaine in liver disease: a review on molecular mechanisms. Eur. J. Pharmacol. 2021;912 doi: 10.1016/j.ejphar.2021.174604. [DOI] [PubMed] [Google Scholar]
- Wang L.J., Zhang H.W., Zhou J.Y., Liu Y., Yang Y., Chen X.L., Zhu C.H., Zheng R.D., Ling W.H., Zhu H.L. Betaine attenuates hepatic steatosis by reducing methylation of the MTTP promoter and elevating genomic methylation in mice fed a high-fat diet. J. Nutr. Biochem. 2014;25:329–336. doi: 10.1016/j.jnutbio.2013.11.007. [DOI] [PubMed] [Google Scholar]
- Werge M.P., McCann A., Galsgaard E.D., Holst D., Bugge A., Albrechtsen N.J.W., Gluud L.L. The role of the transsulfuration pathway in non-alcoholic fatty liver disease. J. Clin. Med. 2021;10:1081. doi: 10.3390/jcm10051081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L., Huang D., Hu Q., Wu J., Wang Y., Feng J. Betaine alleviates hepatic lipid accumulation via enhancing hepatic lipid export and fatty acid oxidation in rats fed with a high-fat diet. Br. J. Nutr. 2015;113:1835–1843. doi: 10.1017/S0007114515001130. [DOI] [PubMed] [Google Scholar]
- Zhang H., Yan S., Khambu B., Ma F., Li Y., Chen X., Martina J.A., Puertollano R., Li Y., Chalasani N., Yin X.M. Dynamic MTORC1-TFEB feedback signaling regulates hepatic autophagy, steatosis and liver injury in long-term nutrient oversupply. Autophagy. 2018;14:1779–1795. doi: 10.1080/15548627.2018.1490850. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.