Figure 5.
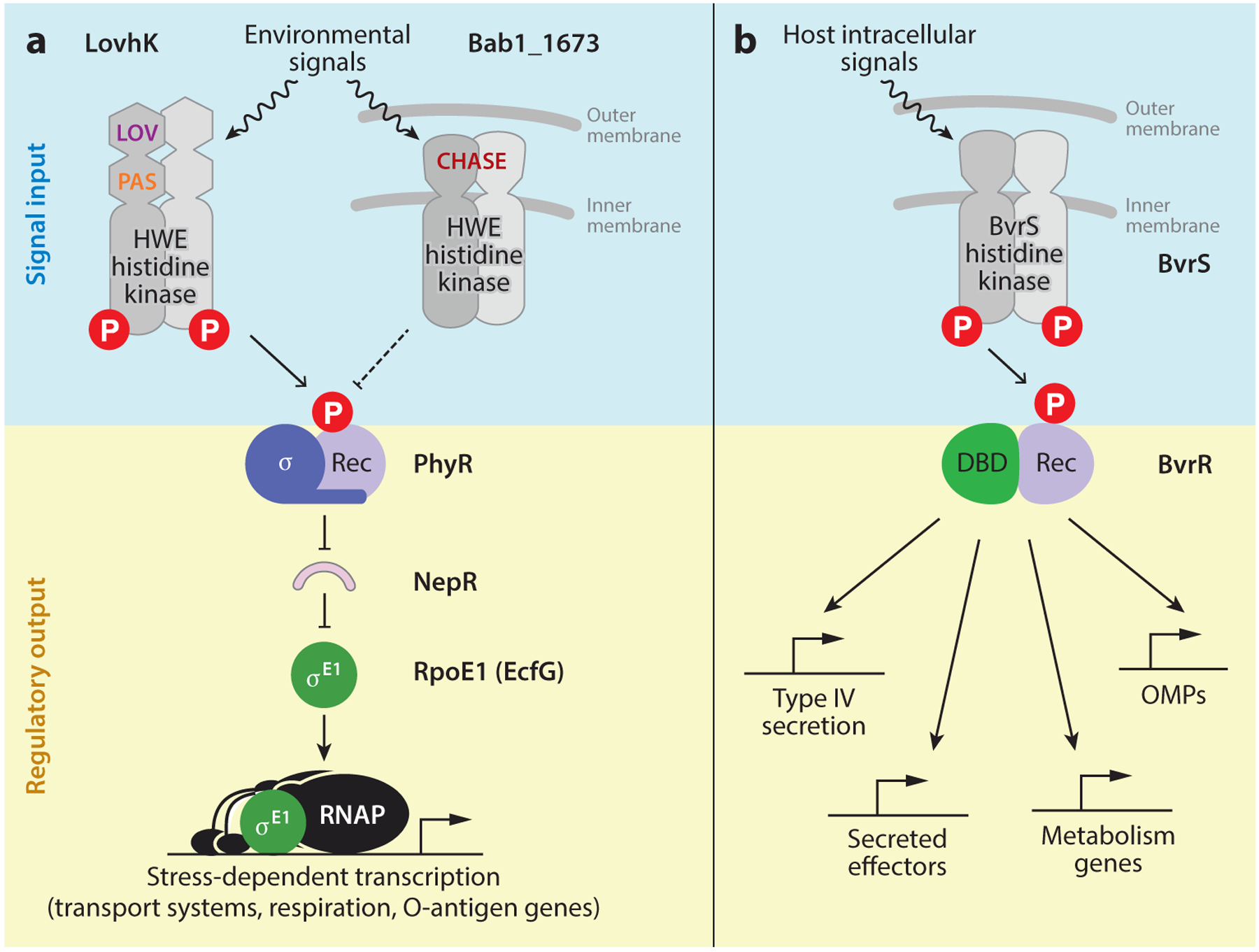
(a) The GSR signaling pathway in Alphaproteobacteria is activated by environmental signals and integrates features of two-component and sigma factor–dependent regulation of gene expression. Studies of this pathway in Brucella abortus support a model in which phosphorylation of PhyR is controlled by two HWE-family sensor kinases: LovhK and Bab1_1673. PhyR phosphorylation promotes its binding to NepR, which releases σE1 to control transcription of select cell envelope genes that influence in vitro stress survival and host infection. The LOV and PAS sensory domains of the cytoplasmic GSR-activating kinase, LovhK, are labeled. The periplasmic CHASE family sensor domain of the transmembrane GSR-repressive kinase, Bab1_1673, is labeled. Solid lines indicate a direct interaction; dashed lines indicate interactions that may be direct or indirect. (b) Brucella spp. BvrR/BvrS is a conserved, archetypal two-component system that is a critical regulator of infection. In response to detection of intracellular signals during infection, the histidine kinase BvrS phosphorylates BvrR, which then regulates transcription of a suite of cell envelope and metabolic genes important for intracellular survival and replication. Abbreviations: DBD, DNA-binding domain; GSR, general stress response; REC, REC, receiver domain; RNAP, RNA polymerase; OMP, outer membrane protein.
