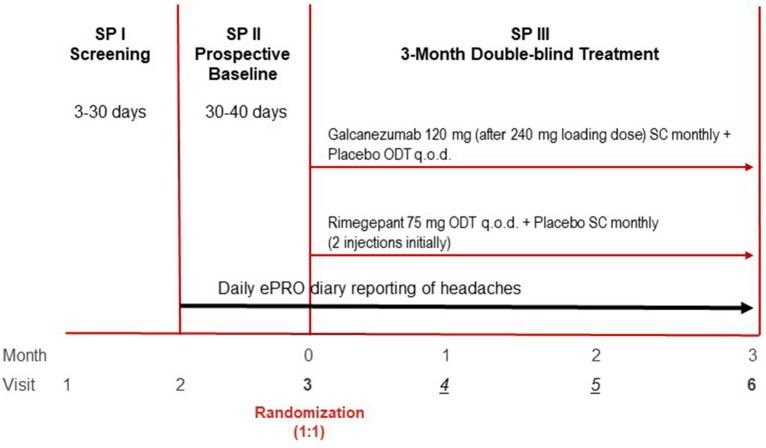
Fig. 1.
Study design. All participants received SC injections using a prefilled syringe and ODT. Visits 1, 2, 3 (randomization) and 6 were conducted in the office. Visits 4 and 5 were telephone visits. At visit 3, participants randomized to galcanezumab 120 mg received a 240-mg loading dose (two injections) and one ODT placebo, and participants randomized to rimegepant received one rimegepant 75 mg ODT and two placebo injections. ePRO electronic patient-reported outcome, ODT orally disintegrating tablet, QOD every other day, SC subcutaneous, SP study period