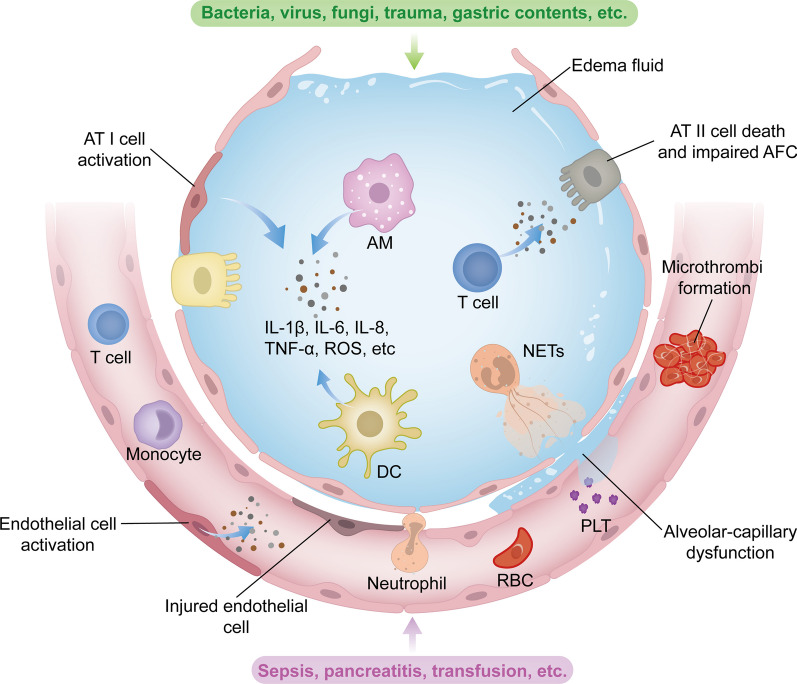
Fig. 1.
Pathophysiology of ARDS. The pathophysiology of ARDS is complex, involving dysregulation of inflammation, alveolar-capillary injury, impaired alveolar fluid clearance and oxidative stress. In case of pulmonary causes such as pneumonia or aspiration, the alveolar epithelium can be directly injured or affected by inducing inflammation. Activation of alveolar epithelial cells, resident alveolar macrophages AMs and dendritic cells DCs leads to the production of inflammatory cytokines and chemokines, recruiting circulating innate and adaptive immune cells into the airspaces. These immune cells amplify inflammation by releasing additional inflammatory molecules. Neutrophils, upon migration, release cytotoxic factors such as ROS and NETs, contributing to the disruption of alveolar-capillary barrier. Endothelial injury due to inflammation activates procoagulant pathways and results in microthrombi formation. These effects ultimately lead to alveolar–capillary barrier injury, allowing the leakage of protein-rich fluid from vasculature into interstitial space and alveoli. The filling of airspaces with edema fluid causes hypoxemia and hypercapnia, which, in turn, reduce fluid and ion clearance. In cases of extrapulmonary ARDS, lung injury is derived from pulmonary endothelial cells. Similarly, pulmonary endothelial dysfunction triggered by circulating injurious molecules can induce inflammatory injury toward the alveolar epithelium, resulting in increased permeability and alveolar oedema. AFC, alveolar fluid clearance; AM, alveolar macrophage; AT I, alveolar type I cell; AT II, alveolar type II cell; DC, dendritic cell; IL-1β, interleukin-1β; IL-6, interleukin-6; IL-8, interleukin-8; NETs, neutrophil extracellular traps; PLT, platelet; RBC, red blood cell; ROS, reactive oxygen species; TNF-α, tumor necrosis factor-α