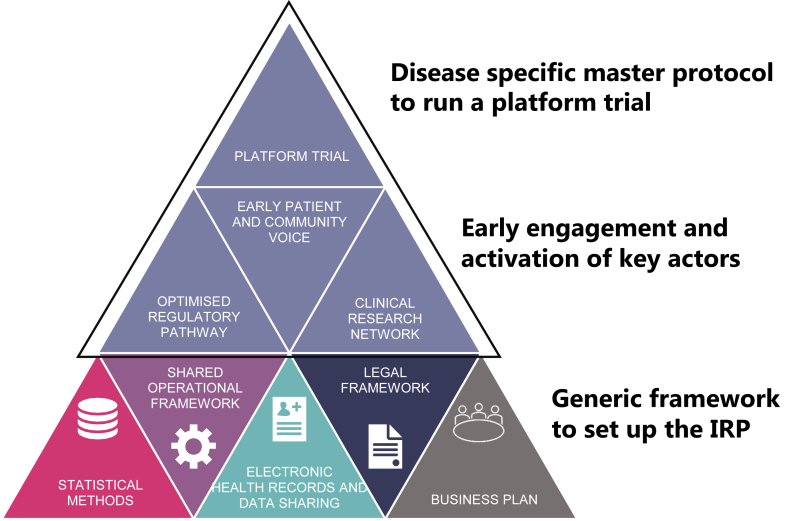
Fig. 2.
Elements of Integrated Research Platforms for drug development in the context of EU-PEARL. Three different layers describe the foundation which should be put in place and are the basis to conduct a platform trial for a certain disease. Layer one encompasses the generic framework to develop a platform trial, which are the same in any Integrated Research Platform (IRP) or disease. Layer two indicates the stakeholders which should be engaged in the building of the IRP very early but are disease specific. On top is the disease specific master protocol for the platform trial, e.g., in EU-PEARL both generic templates and (illustrated by the shared operational framework) and disease specific templates (top circle) have been developed for major depressive disorder (MDD), tuberculosis (TB), non-alcoholic steatohepatitis (NASH), and neurofibromatosis (NF).