Abstract
Background
Elevated homocysteine levels have been shown to be an independent risk factor for cardiovascular disease. However studies of homocysteine lowering in general and end‐stage kidney disease (ESKD) populations have not demonstrated a reduction in cardiovascular event rates. Kidney transplant recipients have high homocysteine levels, high cardiovascular event rates and, unlike the ESKD population, may achieve normalisation of homocysteine levels with homocysteine lowering therapies. Thus may benefit from homocysteine lowering therapy.
Objectives
To evaluate the effects of established homocysteine lowering therapy on cardiovascular mortality in patients with functioning kidney transplants.
Search methods
We searched the Cochrane Renal Group's Specialised Register to 16 March 2015 through contact with the Trials' Search Co‐ordinator using search terms relevant to this review.
Selection criteria
Randomised controlled trials of any therapy that has been shown to significantly lower homocysteine levels conducted in people with functioning kidney transplants. Studies were to be included if they compared homocysteine lowering therapy with placebo or usual care, or compare higher versus lower doses of homocysteine lowering therapy.
Data collection and analysis
Two authors independently assessed study quality and extracted data. Results were to be expressed as the risk ratio (RR) for dichotomous outcomes or mean difference (MD) for continuous outcomes with 95% confidence intervals (CI). Data was to be pooled using the random effects model.
Main results
The literature search yielded 359 reports of which only one study was identified that met our inclusion criteria and reported relevant clinical endpoints. This study randomised 4110 adult participants with a functioning kidney transplant and elevated homocysteine levels to folic acid plus high dose B multivitamins or low dose multivitamins who were followed for a mean 4.0 years. Despite effectively lowering homocysteine levels) in homocysteine levels at follow‐up (MD ‐4.40 μmol/L, 95% CI ‐5.98 to ‐2.82) there was no evidence the intervention impacted on any of the outcomes reported including cardiovascular mortality (RR 0.91, 95% CI 0.69 to 1.20), all‐cause mortality (RR 1.04, 95% CI 0.88 to 1.22), myocardial infarction (RR 1.02, 95% CI 0.77 to 1.35), stroke (RR 1.08, 95% CI 0.69 to 1.71), commencement of renal replacement therapy (RR 1.12, 95% CI 0.91 to 1.37) or all reported adverse events (RR 1.02, 95% CI 0.87 to 1.20). There was no evidence the intervention impacted on the primary endpoint of the study, a cardiovascular event composite (RR 0.99, 95% CI 0.85 to 1.15). The study was of high quality.
Authors' conclusions
There is no current evidence to support the use of homocysteine lowering therapy for cardiovascular disease prevention in kidney transplant recipients.
Plain language summary
Interventions for lowering plasma homocysteine levels in kidney transplant recipients
People with high homocysteine levels have higher rates of cardiovascular disease than those with homocysteine levels within the normal range. Kidney transplant recipients have proportionately more cardiovascular disease events than the general population. The aim of this review was to determine if homocysteine lowering therapies effectively reduce cardiovascular event rates in kidney transplant recipients. A single study was identified that randomised 4110 adult participants with a functioning kidney transplant to homocysteine lowering with folic acid and high dose multivitamins or to low dose multivitamins and followed them for an average of four years. Despite effectively lowering homocysteine levels, there was no evidence of benefit for any of a range of cardiovascular events. Similarly there was no evidence of harm.
Background
Description of the condition
Kidney transplantation is the treatment of choice for end‐stage kidney disease (ESKD), producing a life changing improvement in quality of life and adding approximately 10 years to the life expectancy of patients with ESKD on the transplant waiting list (NIH 2007). Despite the many developments in kidney transplantation over the last 50 years, recipients of kidney transplants continue to have an excess mortality and morbidity compared with the general population (NIH 2007). Cardiovascular disease (CVD) is a leading cause of death and late graft loss in kidney transplant recipients (Kasiske 1996; NIH 2007). In a recent report of a RCT in kidney transplant recipients with 20 years follow‐up, cardiovascular deaths accounted for 53% of the total death rate (Gallagher 2009). Similar findings were reported by the large Assessment of Lescol in Renal Transplantation (ALERT) study (ALERT Study 2003). An observational cohort study has also reported the cumulative incidence of CVD 15 years after transplantation to be 23% for coronary artery disease, 15% for cerebrovascular disease and 15% for peripheral vascular disease (PVD) (Kasiske 1996). The overall risk of CVD following kidney transplantation is five times higher than that of the general population (Kasiske 1996).
Description of the intervention
In untreated classical homocysteinuria, a homozygous genetic disorder of C677T MTHFR resulting in very high levels of plasma homocysteine (100 to 400 μmol/L), death at a young age from venous thromboembolism and malignant arterial disease is frequently observed. Moreover, long‐term treatments that lower homocysteine levels have been extremely effective in reducing the potentially life threatening vascular risk of these patients (Yap 2003). In addition, in the general population and Kidney transplant recipients high homocysteine levels has been shown to be an independent risk factor for CVD including stroke, myocardial infarction (MI), atherosclerosis, arterial and venous thrombosis and cardiovascular death in the general population (Ducloux 2000; HSC 2002; Massy 1994; Wald 2002). In kidney transplant recipients, every 1 μmol/L increase in total homocysteine is associated with a 6% increase in the risk of developing CVD, including MI, stroke, PVD and death (Ducloux 2000). Furthermore, hyperhomocysteinaemia has also been correlated to kidney allograft loss in kidney transplant recipients (Winkelmayer 2005). The striking benefits achieved in patients with homocysteinuria have long been speculated to also be reproducible in other general, chronic kidney disease (CKD) and kidney transplant recipients populations with elevated homocysteine levels. However interventions that lowered homocysteine levels have not yet been shown to reduce cardiovascular risk in either the CKD (Jamison 2007; Vianna 2007; Wrone 2004; Zoungas 2006) or in the general population (Albert 2008; Bonaa 2006; Lonn 2006; Schnyder 2002; Toole 2004).
How the intervention might work
Homocysteine is thought to play an active role in the pathogenesis of atherosclerosis by damaging the endothelium and promoting intra‐arterial and venous thrombosis. There is strong experimental evidence that hyperhomocysteinaemia produces endothelial cell injury and proliferation of medial smooth muscle cells (Lang 2000; Lentz 1996; McCully 1996; Starkebaum 1986). In addition homocysteine has been found to enhance the activity of and increase the synthesis of clotting factors (D'Angelo 1997; Lentz 1991).
Why it is important to do this review
The role of homocysteine lowering in kidney transplant recipients has not been established. The kidney transplant recipient group may be the ideal group to test the homocysteine hypothesis as they have a high cardiovascular event rate (Kasiske 1996) and unlike the ESKD population, can achieve normal homocysteine levels with folic acid, vitamin B12, and vitamin B6 treatment (Beaulieu 1999).
The harms of homocysteine lowering interventions have also not been established. Whilst it is generally believed that folic acid, vitamin B6 and B12 supplementation are safe, there are concerns that high folic acid levels may lead to increased cancer risk (Hubner 2007). This is of particular concern in the kidney transplant recipient group as they have higher absolute rates of malignancy than the general population. Thus even a small increase in relative risk of cancer may outweigh any potential benefits.
Efforts to reduce cardiovascular risk in kidney transplant recipients are attractive because of the large potential benefit of treatment. The European clinical guidelines (EBPG 2002) state the need for more research to be conducted as there is no evidence that reduction of homocysteine levels decreases the incidence of CVD in kidney transplant recipients.
This meta‐analysis aims to assess the benefits and harms of homocysteine lowering therapy in kidney transplant recipients in order to guide decision making and improve outcomes for this patient population.
Objectives
To evaluate the effects of established homocysteine lowering therapy on cardiovascular mortality in patients with functioning kidney transplants.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) and quasi‐RCTs (allocation to treatment was obtained by alteration, use of alternate medical records, date of birth or other predictable methods).
Including a minimum of 100 patient‐years follow‐up (to reduce the risk of reporting or publication bias).
Studies with a sequential or cross‐over design were excluded.
Types of participants
All patients (adults and children) with a functioning kidney transplant defined as a kidney transplant in situ with no requirement for maintenance dialysis, or as defined by study authors.
Types of interventions
Studies randomising patients to any therapy which has been shown to significantly lower homocysteine levels were included (e.g. folic acid, vitamin B6 and vitamin B12). Studies of regimens in which a major mechanism of action is not thought to be homocysteine lowering will be excluded (e.g. simvastatin plus folic acid). Comparisons to be investigated were as follows.
Homocysteine lowering therapy versus placebo or usual care
Higher versus lower dose homocysteine lowering therapy
Any schedule of treatment
Any route of treatment.
Types of outcome measures
Primary outcomes
Cardiovascular mortality
Secondary outcomes
All‐cause mortality
-
Cardiovascular disease
Fatal and nonfatal MI
Coronary revascularization
-
Cerebrovascular disease
Stroke
Cerebrovascular revascularization
-
PVD and venous thromboembolic disease
Lower limb amputation
Deep vein thrombosis (DVT) and pulmonary embolism (PE)
-
Kidney‐specific outcomes
Commencement of renal replacement therapy (RRT) (dialysis or transplantation)
Change in kidney function
-
Adverse events from folic‐based therapy
Gastrointestinal events
Dermatological events
Neurological events
Malignancy incidence and mortality
Any self‐reported adverse events
Search methods for identification of studies
Electronic searches
We searched the Cochrane Renal Group's Specialised Register to 16 March 2015 through contact with the Trials' Search Co‐ordinator using search terms relevant to this review. The Cochrane Renal Group’s Specialised Register contains studies identified from the following sources.
Monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL)
Weekly searches of MEDLINE OVID SP
Handsearching of renal‐related journals and the proceedings of major renal conferences
Searching of the current year of EMBASE OVID SP
Weekly current awareness alerts for selected renal journals
Searches of the International Clinical Trials Register (ICTRP) Search Portal and ClinicalTrials.gov.
Studies contained in the Specialised Register are identified through search strategies for CENTRAL, MEDLINE, and EMBASE based on the scope of the Cochrane Renal Group. Details of these strategies, as well as a list of handsearched journals, conference proceedings and current awareness alerts, are available in the specialised register section of information about the Cochrane Renal Group.
See Appendix 1 for search terms used in strategies for this review.
Searching other resources
Reference lists of clinical practice guidelines, review articles and relevant studies.
Experts in the field were contacted for additional studies.
Data collection and analysis
Selection of studies
Two authors independently assessed each reference for eligibility. Language was not an exclusion criterion. Disagreement regarding inclusion in the review was resolved by consensus among three authors.
Data extraction and management
Data extraction was performed independently by two authors using a standardised data form, who independently entered the data into RevMan 5. Where more than one publication of the study exists, the publications with the most complete data will be included. Where relevant outcomes were only published in earlier versions, these data were to be used. Any discrepancy between published versions was to be noted. The original author was to be contacted via written correspondence for any further information or clarification of unclear data. Disagreements were to be resolved by consensus among three authors.
Assessment of risk of bias in included studies
Two authors were to independently assess the following items using the risk of bias assessment tool (Higgins 2011) (see Appendix 2).
Was there adequate sequence generation (selection bias)?
Was allocation adequately concealed (selection bias)?
-
Was knowledge of the allocated interventions adequately prevented during the study (detection bias)?
Participants and personnel
Outcome assessors
Were incomplete outcome data adequately addressed (attrition bias)?
Are reports of the study free of suggestion of selective outcome reporting (reporting bias)?
Was the study apparently free of other problems that could put it at a risk of bias?
Measures of treatment effect
For dichotomous outcomes (all‐cause mortality, MI, coronary revascularization, cardiovascular death, stroke, cerebrovascular revascularization, lower limb amputation, DVT, PE, commencement of RRT), results were to be expressed as risk ratio (RR) with 95% confidence intervals (CI).
If a significant risk reduction was found, the absolute risk reduction with therapy was to be calculated in relation to the absolute risk found in the placebo/comparator group.
Dealing with missing data
Where outcomes sought were reported in insufficient detail to allow meta‐analysis and further information was not forthcoming from triallists, these outcomes were to be tabulated and assessed with descriptive techniques and where possible the risk difference (RD) with 95% CI was to be calculated.
If sufficient RCTs were identified, an attempt was to be made to evaluate the risk of publication bias using a funnel plot. Attrition bias was to be assessed using the loss/event ratio.
Assessment of heterogeneity
Heterogeneity was to be analysed using a Chi2 test on N‐1 degrees of freedom, with an alpha of 0.05 used for statistical significance and with the I2 test (Higgins 2003). I2 values of 25%, 50% and 75% were taken to correspond to low, medium and high levels of heterogeneity.
Assessment of reporting biases
The intention was that the risk of publication bias was to be evaluated using a funnel plot. Attrition bias was to be assessed using the loss/event ratio.
Data synthesis
The intention was that data was to be pooled using the random‐effects model but the fixed‐effect model would also be analysed to ensure robustness of the model chosen and susceptibility to outliers.
Subgroup analysis and investigation of heterogeneity
Subgroup analyses were to be conducted to explore possible sources of heterogeneity. Heterogeneity was to be analysed using the Cochran Q test on N‐1 degrees of freedom, with P < 0.05 used to denote statistical significance, and the I2 test (with uncertainty intervals). Subgroup analyses were to be conducted according to the following characteristics.
Gender
Adults and children
History of cardiac disease or diabetes mellitus
Prior vitamin supplementation
Concurrent vitamin supplementation
Concomitant medications (e.g. aspirin)
Mandatory grain fortification in the country study conducted
Baseline homocysteine level (≤ upper limit normal (ULN) versus > ULN).
We intended to conduct a subgroup analysis if possible using these characteristics. Plausible explanations for variations in treatment effect were to be explored using subgroup analyses based on study quality and length of follow‐up.
Sensitivity analysis
Sensitivity analyses were to be conducted to ensure conclusions were robust to decisions made during the review process such as inclusion criteria and imputing of missing data. Sensitivity analyses were also to be conducted to assess the influence of methodological quality.
Results
Description of studies
Results of the search
The literature search yielded a total of 359 records (Figure 1). Of these, 44 were reviewed in full text. One study (13 reports) was identified that met our inclusion criteria (FAVORIT Study 2006).
1.
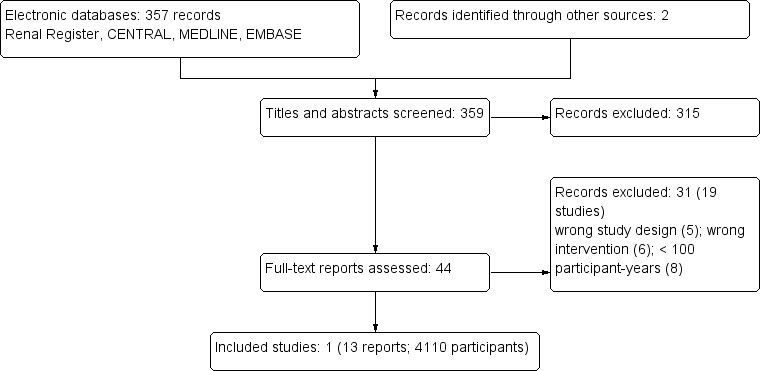
Study flow diagram
Included studies
Participants
The study randomised 4410 people aged 35 to 75 years with a functioning kidney transplant who were at least six months post‐transplantation with stable kidney function and an elevated homocysteine level (≥ 11 µmol/L women; ≥ 12 µmol/L men). The mean follow‐up time was 4.0 years.
Roughly one third (37.2%) were female, one quarter (23.5%) were of non‐white race, one fifth had a history of cardiovascular disease (20.0%) and two fifths had diabetes mellitus (40.5%). Participants were recruited from the US (73%), Brazil (14.9%) and Canada (12.1%) between August 2002 and January 2007. The vast majority of participants would have been recruited during the era of mandatory grain fortification with folic acid which was introduced in 1998 in the USA and Canada (Crider 2011) and in June 2004 in Brazil (Orioli 2011). Patients had functioning transplants for an average of 5 ± 5.0 years standing with an average screening eGFR of 48.8 ± 16.2 mL/min. Mean homocysteine levels were 16.4 ± 1.3 mmol/L.
Interventions
The intervention was folic acid 5.0 mg plus high (50 mg vitamin B6; 1.0 mg vitamin B12) or low (1.3 mg vitamin B6; 2.0 µg vitamin B12) dose multivitamins.
Outcomes
The primary outcome was a composite of cardiovascular disease (cardiovascular death, MI, resuscitated sudden death, stroke, coronary artery revascularization, lower extremity revascularization, above‐ankle amputation for severe arterial disease, carotid endarterectomy or angioplasty, abdominal aortic aneurysm repair or renal artery revascularization). Patients commencing dialysis continued on study treatment until they reached a primary endpoint whereupon study medication was ceased.
Excluded studies
After full text review we excluded 31 records (19 studies). The reasons for exclusion were: wrong study design (5); wrong intervention (6) or < 100 patient‐years. See Characteristics of excluded studies.
Risk of bias in included studies
The identified study has an overall low risk of bias (Risk of bias in included studies).
Effects of interventions
Meta‐analysis was not applied as only a single eligible study was identified (FAVORIT Study 2006).
FAVORIT Study 2006 found that, based on a subgroup of 143 participants, high dose folic acid and B group vitamins significantly lowered homocysteine levels (Analysis 1.1 (143 participants): ‐4.40 μmol/L, 95% CI ‐5.98 to ‐2.82).
1.1. Analysis.
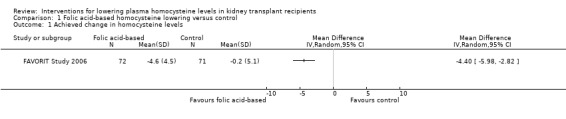
Comparison 1 Folic acid‐based homocysteine lowering versus control, Outcome 1 Achieved change in homocysteine levels.
Despite effectively lowering homocysteine levels there was no evidence the intervention impacted on any of the outcomes for this review.
Cardiovascular mortality (Analysis 1.2 (4110 participants): RR 0.91, 95% CI 0.69 to 1.20)
All‐cause mortality (Analysis 1.3 (4110 participants): RR 1.04, 95% CI 0.88 to 1.22)
MI (Analysis 1.4 (4110 participants): RR 1.02, 95% CI 0.77 to 1.35)
Coronary revascularization (Analysis 1.5 (4110 participants): RR 0.93, 95% CI 0.73 to 1.19)
Stroke (Analysis 1.6 (4110 participants): RR 1.08, 95% CI 0.69 to 1.71)
Cerebrovascular revascularization (defined in the FAVORIT Study 2006 as carotid endarterectomy or angioplasty) (Analysis 1.7 (4110 participants): RR 1.11, 95% CI 0.45 to 2.73)
Commencement of RRT (defined in the FAVORIT Study 2006 as dialysis‐dependent kidney failure) (Analysis 1.8 (4110 participants): RR 1.12, 95% CI 0.91 to 1.37)
Adverse gastrointestinal events (Analysis 1.9 (4110 participants): RR 1.06, 95% CI 0.83 to 1.36)
All reported adverse events (Analysis 1.10 (4110 participants): RR 1.02, 95% CI 0.87 to 1.20).
1.2. Analysis.
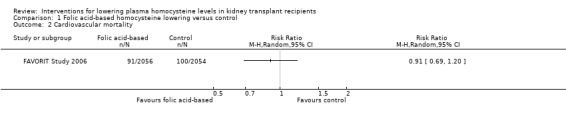
Comparison 1 Folic acid‐based homocysteine lowering versus control, Outcome 2 Cardiovascular mortality.
1.3. Analysis.
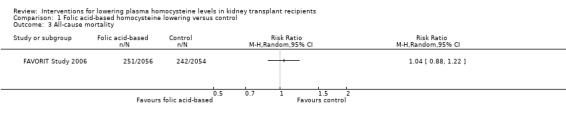
Comparison 1 Folic acid‐based homocysteine lowering versus control, Outcome 3 All‐cause mortality.
1.4. Analysis.
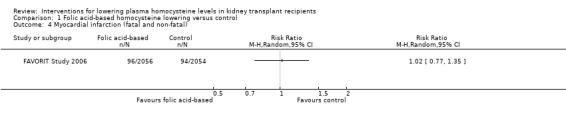
Comparison 1 Folic acid‐based homocysteine lowering versus control, Outcome 4 Myocardial infarction (fatal and non‐fatal).
1.5. Analysis.
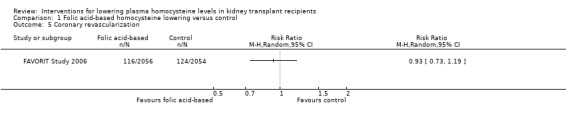
Comparison 1 Folic acid‐based homocysteine lowering versus control, Outcome 5 Coronary revascularization.
1.6. Analysis.
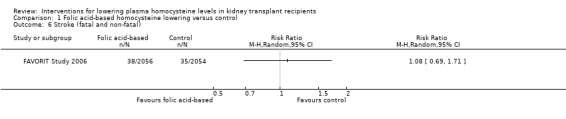
Comparison 1 Folic acid‐based homocysteine lowering versus control, Outcome 6 Stroke (fatal and non‐fatal).
1.7. Analysis.
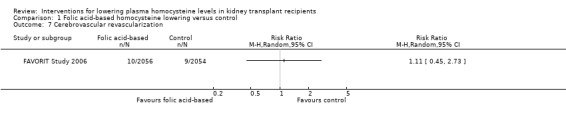
Comparison 1 Folic acid‐based homocysteine lowering versus control, Outcome 7 Cerebrovascular revascularization.
1.8. Analysis.
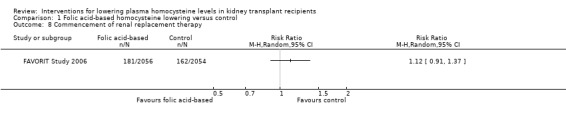
Comparison 1 Folic acid‐based homocysteine lowering versus control, Outcome 8 Commencement of renal replacement therapy.
1.9. Analysis.
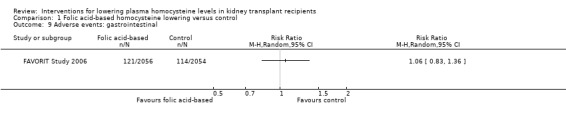
Comparison 1 Folic acid‐based homocysteine lowering versus control, Outcome 9 Adverse events: gastrointestinal.
1.10. Analysis.
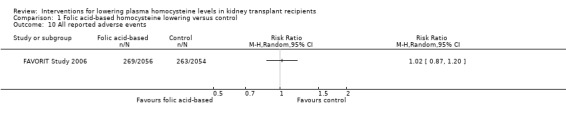
Comparison 1 Folic acid‐based homocysteine lowering versus control, Outcome 10 All reported adverse events.
No data were reported in the FAVORIT Study 2006 for change in kidney function, deep vein thrombosis and PE, lower limb amputation per se (although it was included in a PVD composite), adverse dermatological events, adverse neurological events or adverse malignant events.
There was no evidence the intervention impacted on the primary endpoint of the FAVORIT Study 2006, a cardiovascular event composite (RR 0.99, 95% CI 0.85 to 1.15), nor on any of the secondary endpoints not mentioned above including resuscitated sudden death (RR 0.80, 95% CI 0.32 to 2.02), PVD defined as lower extremity revascularization or amputation above the ankle for severe arterial disease (RR 1.17, 95% CI 0.81 to 1.67), abdominal aortic aneurysm repair (RR 0.60, 95% CI 0.14 to 2.50) and renal artery revascularization (RR 1.28, 95% CI 0.48 to 3.44).
Discussion
Summary of main results
This review identified only one completed study that met our inclusion criteria for examining the effectiveness of homocysteine lowering in kidney transplant recipients. In this study, there was no evidence that homocysteine lowering had an effect on any of the assessed cardiovascular outcomes, including cardiovascular mortality, MI, and stroke, other clinical outcomes, including all‐cause mortality, requirement for dialysis treatment or access thrombosis, nor on adverse effects.
Overall completeness and applicability of evidence
Beyond kidney transplantation, the impact of homocysteine has been studied in people with other categories of kidney disease. A systematic review performed by our group examined the impact of folic acid‐based homocysteine lowering in people with any type of kidney disease categorised as ESKD, CKD and functioning kidney transplantation (Jardine 2012). Eleven studies were identified reporting 3045 cardiovascular events among 10,863 participants of which the FAVORIT Study 2006 contributed 4110 participants. There was no evidence homocysteine lowering reduced the primary cardiovascular composite endpoint either overall (RR 0.97, 95% CI 0.92 to 1.03) nor in any of three defined categories of kidney disease (P = 0.785). This data is consistent with studies in the general population, where folic acid based homocysteine lowering has also not been found to prevent cardiovascular events in large RCTs. The B‐Vitamin Treatment Trialists’ Collaboration has performed two individual patient level data analyses of larger studies randomising participants to folate‐containing B group vitamins (Clarke 2010; Vollset 2013) although neither were able to include the FAVORIT Study 2006. The first primarily analysed the impact on the incidence of vascular disease in 37,485 participants in eight studies while the second assessed cancer incidence in 49,621 participants in 13 studies. Over a median of five years of treatment, folate‐containing B group vitamin supplementation had no impact on major vascular events (RR 1.01, 95% CI 0.97 to 1.05) or mortality (RR 1.02, 95% CI 0.97 to 1.08) despite an average 25% reduction in homocysteine levels. There was no evidence of heterogeneity in subgroup analyses comparing the impact of the intervention according to serum creatinine (< 80, 80 to 94 and ≥ 95 µmol/L). Similarly there was no impact on cancer incidence over average five years treatment duration (RR 1.06, 95% CI 0.99 to 1.13). In combination these studies appear to have effectively excluded any beneficial cardiovascular effect of homocysteine lowering therapy in the general population and in people with kidney disease.
Quality of the evidence
The included study (FAVORIT Study 2006) was of assessed as high quality.
Potential biases in the review process
We specifically included only RCTs with a minimum of 100 patient‐years follow‐up in our inclusion criteria to reduce the risk of reporting or publication bias that may be associated with small studies (Egger 1997). To investigate the impact of the 100 patient‐year criteria on our results, we modified our inclusion criteria to include studies of any follow‐up duration that met all other search criteria in a sensitivity analysis. Excluding the 100 patient‐year minimum requirement resulted in identification of an extra six studies (Beaulieu 1999; Biagini 2002; Bostom 1997; Marcucci 2002; Perez 2004; Xu 2005a).The intervention used in these studies was either folic acid or folic acid, vitamin B6 and vitamin B12. Follow‐up ranged from three to 30 patient‐years. Baseline homocysteine levels ranged from 17 to 30 µmol/L (compared with levels of 100 to 400 μmol/L reported in classical homocysteinuria). Four studies found a significant decrease in fasting homocysteine levels with treatment compared with placebo/lower dose (Marcucci 2002, Beaulieu 1999, Xu 2005a, Bostom 1997). Perez 2004 compared standard and supraphysiological doses of folic acid, vitamin B6 and vitamin B12 and found no significant difference in homocysteine levels between the groups. Some of these studies did not report baseline and achieved homocysteine levels for each group, which prevented their combination using meta‐analysis (Bostom 1997; Perez 2004; Xu 2005a). Marcucci 2002 reported a significant decrease in carotid intima‐media thickness (cIMT) in the treatment arm (0.95 ± 0.20 mm versus 0.64 ± 0.17 mm; P < 0.0001) and an increase in cIMT in the placebo group (0.71 ± 0.16 mm versus 0.87 ± 0.19 mm; P < 0.05). Xu 2005a found a significant increase in endothelium dependent and independent vasodilatation response following the intervention (12.2% ± 4.6% versus 8.8% ± 5.2%, t = 2.9, P < 0/01 and 17.6% ± 3.9% versus 12.2% ± 4.7%, t = 3.4, P < 0.01) and there were no significant changes observed in controls. None of these RCTs reported the defined clinical events and therefore could not contribute to our planned analyses. Therefore, regardless of the patient‐year parameter in our inclusion criteria, we were unable to find more than one completed study that evaluated the effect of homocysteine lowering therapy on cardiovascular end points rather than surrogate markers for cardiovascular disease.
Agreements and disagreements with other studies or reviews
The KDIGO 2009 and CARI 2012 for the care of people with functioning kidney transplants do not comment on folic acid or B vitamin supplementation. The UK Renal Association suggests offering folic acid and B group vitamin supplementation to patients with kidney disease considered at risk of nutritional deficiency but notes insufficient evidence to recommend supraphysiological supplementation for vascular risk modification (The Renal Association 2010). The guidelines noted the (then) ongoing FAVORIT Study 2006 would supply evidence for people with functioning kidney transplants.
Authors' conclusions
Implications for practice.
There is no current evidence to support the use of homocysteine lowering therapy for cardiovascular disease prevention in kidney transplant recipients.
Implications for research.
Research focusing on mechanisms to reduce cardiovascular disease events in kidney transplant recipients is warranted.
Acknowledgements
We would like to thank the Cochrane Renal Group for their help and support.
We are indebted to Ms Gail Higgins, Trials Search Co‐ordinator of the Cochrane Renal Group for assistance with the search strategy and implementation.
We would like to thank Drs Conal Daly, Richard Haynes and David Wheeler for their editorial advice during the preparation of this review.
Appendices
Appendix 1. Electronic search strategies
| Databases | Search terms |
| CENTRAL |
|
| MEDLINE |
|
| EMBASE |
|
Appendix 2. Risk of bias assessment tool
| Potential source of bias | Assessment criteria |
|
Random sequence generation Selection bias (biased allocation to interventions) due to inadequate generation of a randomised sequence |
Low risk of bias: Random number table; computer random number generator; coin tossing; shuffling cards or envelopes; throwing dice; drawing of lots; minimization (minimization may be implemented without a random element, and this is considered to be equivalent to being random). |
| High risk of bias: Sequence generated by odd or even date of birth; date (or day) of admission; sequence generated by hospital or clinic record number; allocation by judgement of the clinician; by preference of the participant; based on the results of a laboratory test or a series of tests; by availability of the intervention. | |
| Unclear: Insufficient information about the sequence generation process to permit judgement. | |
|
Allocation concealment Selection bias (biased allocation to interventions) due to inadequate concealment of allocations prior to assignment |
Low risk of bias: Randomisation method described that would not allow investigator/participant to know or influence intervention group before eligible participant entered in the study (e.g. central allocation, including telephone, web‐based, and pharmacy‐controlled, randomisation; sequentially numbered drug containers of identical appearance; sequentially numbered, opaque, sealed envelopes). |
| High risk of bias: Using an open random allocation schedule (e.g. a list of random numbers); assignment envelopes were used without appropriate safeguards (e.g. if envelopes were unsealed or non‐opaque or not sequentially numbered); alternation or rotation; date of birth; case record number; any other explicitly unconcealed procedure. | |
| Unclear: Randomisation stated but no information on method used is available. | |
|
Blinding of participants and personnel Performance bias due to knowledge of the allocated interventions by participants and personnel during the study |
Low risk of bias: No blinding or incomplete blinding, but the review authors judge that the outcome is not likely to be influenced by lack of blinding; blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken. |
| High risk of bias: No blinding or incomplete blinding, and the outcome is likely to be influenced by lack of blinding; blinding of key study participants and personnel attempted, but likely that the blinding could have been broken, and the outcome is likely to be influenced by lack of blinding. | |
| Unclear: Insufficient information to permit judgement | |
|
Blinding of outcome assessment Detection bias due to knowledge of the allocated interventions by outcome assessors. |
Low risk of bias: No blinding of outcome assessment, but the review authors judge that the outcome measurement is not likely to be influenced by lack of blinding; blinding of outcome assessment ensured, and unlikely that the blinding could have been broken. |
| High risk of bias: No blinding of outcome assessment, and the outcome measurement is likely to be influenced by lack of blinding; blinding of outcome assessment, but likely that the blinding could have been broken, and the outcome measurement is likely to be influenced by lack of blinding. | |
| Unclear: Insufficient information to permit judgement | |
|
Incomplete outcome data Attrition bias due to amount, nature or handling of incomplete outcome data. |
Low risk of bias: No missing outcome data; reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias); missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk not enough to have a clinically relevant impact on the intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size; missing data have been imputed using appropriate methods. |
| High risk of bias: Reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk enough to induce clinically relevant bias in intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes enough to induce clinically relevant bias in observed effect size; ‘as‐treated’ analysis done with substantial departure of the intervention received from that assigned at randomisation; potentially inappropriate application of simple imputation. | |
| Unclear: Insufficient information to permit judgement | |
|
Selective reporting Reporting bias due to selective outcome reporting |
Low risk of bias: The study protocol is available and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way; the study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified (convincing text of this nature may be uncommon). |
| High risk of bias: Not all of the study’s pre‐specified primary outcomes have been reported; one or more primary outcomes is reported using measurements, analysis methods or subsets of the data (e.g. subscales) that were not pre‐specified; one or more reported primary outcomes were not pre‐specified (unless clear justification for their reporting is provided, such as an unexpected adverse effect); one or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis; the study report fails to include results for a key outcome that would be expected to have been reported for such a study. | |
| Unclear: Insufficient information to permit judgement | |
|
Other bias Bias due to problems not covered elsewhere in the table |
Low risk of bias: The study appears to be free of other sources of bias. |
| High risk of bias: Had a potential source of bias related to the specific study design used; stopped early due to some data‐dependent process (including a formal‐stopping rule); had extreme baseline imbalance; has been claimed to have been fraudulent; had some other problem. | |
| Unclear: Insufficient information to assess whether an important risk of bias exists; insufficient rationale or evidence that an identified problem will introduce bias. |
Data and analyses
Comparison 1. Folic acid‐based homocysteine lowering versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Achieved change in homocysteine levels | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2 Cardiovascular mortality | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3 All‐cause mortality | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4 Myocardial infarction (fatal and non‐fatal) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 5 Coronary revascularization | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 6 Stroke (fatal and non‐fatal) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 7 Cerebrovascular revascularization | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 8 Commencement of renal replacement therapy | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 9 Adverse events: gastrointestinal | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 10 All reported adverse events | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
FAVORIT Study 2006.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Other information
|
|
| Outcomes |
|
|
| Funding source |
|
|
| Presence or absence of grain fortification |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization by permuted block, stratified by clinical site". Two different block sizes were used. |
| Allocation concealment (selection bias) | Low risk | "Randomization ... was performed through the data management system. Because the need for emergency unblinding was expected to be low, unblinding codes were stored securely at the Data Coordinating Center, accessible only to authorized staff." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | This was a placebo‐controlled RCT with both multivitamin preparations formulated to be similar in appearance and smell. Blinding was explicitly tested by survey of participants and study coordinators with 49% of each group providing incorrect guesses of intervention allocation. "The trial was a .. double blind, randomised clinical trial". "Both multivitamins [standard and low dose] were formulated to be similar in appearance and odor to facilitate blinding" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The first 4 components of the primary outcome (cardiovascular death, MI, resuscitated sudden death and stroke) were centrally reviewed and adjudicated. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Completeness of follow‐up: deceased (493); complete follow‐up to June 2009 (2788); incomplete follow‐up to June 2009 (822); no follow‐up (7) Withdrawal of consent: treatment group (198/2056); control group (171/2054) |
| Selective reporting (reporting bias) | Low risk | Event data for all the primary and secondary outcomes according to intention‐to‐treat are reported. |
| Other bias | Low risk | No other biases detected |
CrCl ‐ creatinine clearance; ESKD ‐ end‐stage kidney disease; MI ‐ myocardial infarction; RCT ‐ randomised controlled trial
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ardalan 2003 | Not RCT |
| Austen 2006 | Cross‐over study |
| Beaulieu 1999 | < 100 patient‐years. No clinical events, only plasma homocysteine levels |
| Biagini 2002 | < 100 patient‐years. No clinical events, only carotid intima‐media thickness |
| Bostom 1997 | < 100 patient‐years. No clinical events, only plasma homocysteine levels |
| Bostom 2000 | Not a comparison of homocysteine lowering |
| Jurewicz 2003 | Not a comparison of homocysteine lowering |
| Juskowa 2006 | Not a comparison of homocysteine lowering |
| LANDMARK 2 Study 2009 | Not a comparison of homocysteine lowering |
| Lash 1998 | Not a comparison of homocysteine lowering |
| Manrique 2005 | < 100 patient‐years |
| Marcucci 2002 | < 100 patient‐years. No clinical events, only carotid intima‐media thickness |
| Nafar 2009 | < 100 patient‐years. This study has been terminated according to ClinicalTrials.gov information |
| Perez 2004 | < 100 patient‐years. No clinical events. Only clinical markers such as lipid profile |
| Rymarz 2009 | Sequential or cross‐over design |
| Savaj 2002 | Not RCT |
| Shemin 2001 | Not RCT |
| Teplan 2003b | Not homocysteine lowering (hypoenergetic hypolipidaemic diet and corticosteroids withdrawal) |
| Xu 2005a | < 100 patient‐years. No clinical events. Only plasma homocysteine levels and endothelium dependent and independent vasodilation responses |
Contributions of authors
Draft the protocol: AK, MJ, VP, SN, SZ, AC, SDN, GS, MG, SK, TN
Study selection: AK, MJ, SN
Extract data from studies: AK, SN
Enter data into RevMan: AK, SN
Carry out the analysis: AK, MJ, VP, TN
Interpret the analysis: MJ, AK, VP, SZ, SN, TN, SDN, GS, AC, MG, SK
Draft the final review: AK, MJ, VP, SN, TN, GS, SDN, AC, MG, SK
Disagreement resolution: MJ, VP
Update the review: MJ
Sources of support
Internal sources
-
The George Institute for Global Health, Australia.
Dr Meg Jardine, Dr Sophie Zoungas, Dr Vlado Perkovic, Dr Alan Cass and Dr Martin Gallagher are employed by The George Institute for Global Health
External sources
No sources of support supplied
Declarations of interest
None stated
New
References
References to studies included in this review
FAVORIT Study 2006 {published data only}
- Bostom AG, Carpenter M, Kusek J. Baseline characteristics of the folic acid for vascular outcome reduction in transplantation (FAVOURIT) trial [abstract no: PUB316]. Journal of the American Society of Nephrology 2008;19(Abstracts Issue):883A. [CENTRAL: CN‐00724882] [Google Scholar]
- Bostom AG, Carpenter MA, Hunsicker L, Jacques PF, Kusek JW, Levey AS. Baseline characteristics of participants in the folic acid for vascular outcome reduction in transplantation (FAVORIT) trial [abstract no: 827]. American Journal of Transplantation 2009;9(Suppl 2):429. [Google Scholar]
- Bostom AG, Carpenter MA, Hunsicker L, Jacques PF, Kusek JW, Levey AS, et al. Baseline characteristics of participants in the Folic Acid for Vascular Outcome Reduction in Transplantation (FAVORIT) Trial. American Journal of Kidney Diseases 2009;53(1):121‐8. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostom AG, Carpenter MA, Kusek JW, Hunsicker LG, Jacques P, Levey AS, et al. Homocysteine‐lowering in chronic stable renal transplant recipients: the FAVORIT Trial [abstract no: LB001]. American Society of Nephrology Renal Week; 2009 Oct 27‐Nov 1; San Diego, CA. 2009.
- Bostom AG, Carpenter MA, Kusek JW, Hunsicker LG, Pfeffer MA, Levey AS, et al. Rationale and design of the Folic Acid for Vascular Outcome Reduction In Transplantation (FAVORIT) trial. American Heart Journal 2006;152(3):448.e1‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Bostom AG, Carpenter MA, Kusek JW, Levey AS, Hunsicker L, Pfeffer MA, et al. Homocysteine‐lowering and cardiovascular disease outcomes in kidney transplant recipients: primary results from the Folic Acid for Vascular Outcome Reduction in Transplantation trial. Circulation 2011;123(16):1763‐70. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter M, Bostom A, Hunsicker L, Ivanova A, Kasiske B, Kusek J, et al. Higher systolic blood pressure is associated with increased cardiovascular risk in patients with hypertension in the Folic Acid for Vascular Outcome Reduction in Transplantation trial [abstract]. American Journal of Transplantation 2011;11(Suppl 2):375. [EMBASE: 70406228] [Google Scholar]
- Carpenter MA, Bostom A, Kusek J, Adey D, Cole E, House A, et al. Untreated CVD risk factors in chronic, stable kidney transplant recipients at baseline in the folic acid for vascular outcome reduction (FAVORIT) study [abstract no: PUB271]. Journal of the American Society of Nephrology 2009;20:888A. [CENTRAL: CN‐00740518] [Google Scholar]
- Carpenter MA, John A, Weir MR, Smith SR, Hunsicker L, Kasiske BL, et al. BP, cardiovascular disease, and death in the Folic Acid for Vascular Outcome Reduction in Transplantation Trial. Journal of the American Society of Nephrology 2014;25(7):1554‐62. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter MA, Weir MR, Adey DB, House AA, Bostom AG, Kusek JW. Inadequacy of cardiovascular risk factor management in chronic kidney transplantation ‐ evidence from the FAVORIT study. Clinical Transplantation 2012;26(4):E438‐46. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troen AM, Scott TM, D'Anci KE, Moorthy D, Dobson B, Rogers G, et al. Cognitive dysfunction and depression in adult kidney transplant recipients: baseline findings from the FAVORIT Ancillary Cognitive Trial (FACT). Journal of Renal Nutrition 2012;22(2):268‐76.e1‐3. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner DE, Carpenter MA, Levey AS, Ivanova A, Cole EH, Hunsicker L, et al. Kidney function and risk of cardiovascular disease and mortality in kidney transplant recipients: the FAVORIT trial. American Journal of Transplantation 2012;12(9):2437‐45. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weir MR, Gravens‐Muller L, Costa N, Ivanova A, Manitpisitkul W, Bostom AG, et al. Safety events in kidney transplant recipients: Results from the Folic Acid for Vascular Outcome Reduction in Transplant Trial. Transplantation 2014:e‐pub ahead of print. [PUBMED: 25393158] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Ardalan 2003 {published data only}
- Ardalan MR, Mortazavi M, Etemadi J. Hyperhomocysteinemia and its therapy in renal transplant recipients with combination of vit B6, vit B12 and folic acid [abstract]. Nephrology Dialysis Transplantation 2003;18(Suppl 4):509‐10. [CENTRAL: CN‐00444217] [Google Scholar]
Austen 2006 {published data only}
Beaulieu 1999 {published data only}
- Beaulieu AJ, Gohh RY, Han H, Hakas D, Jacques PF, Selhub J, et al. Enhanced reduction of fasting total homocysteine levels with supraphysiological versus standard multivitamin dose folic acid supplementation in renal transplant recipients. Arteriosclerosis, Thrombosis & Vascular Biology 1999; Vol. 19, issue 12:2918‐21. [MEDLINE: ] [DOI] [PubMed]
- Gohh RY, Beaulieu AJ, Han H, Hakas D, Jacques PF, Selhub J, et al. Enhanced reduction of fasting total homocysteine levels with supraphysiological versus standard multivitamin dose folic acid supplementation in renal transplant recipients (RTR) [abstract]. Journal of the American Society of Nephrology 1999;10(Program & Abstracts):730A. [CENTRAL: CN‐00626082] [DOI] [PubMed] [Google Scholar]
Biagini 2002 {published data only}
- Biagini M, Bertoni E, Marcucci R, Zanazzi M, Rosati A, Fedi S, et al. Vitamin supplementation reduces the progression atherosclerosis in hyperhomocysteinemic renal transplant recipients [abstract]. XIXth International Congress of the Transplantation Society; 2002 Aug 25‐30; Miami, FL. 2002. [CENTRAL: CN‐00415280] [DOI] [PubMed]
Bostom 1997 {published data only}
- Bostom AG, Gohh RY, Beaulieu A, Nadeau MR, Hume AL, Jacques PF, et al. Homocysteine‐lowering treatment of renal transplant recipients [abstract]. Journal of the American Society of Nephrology 1997;8(Program & Abstracts):675A. [CENTRAL: CN‐00444499] [Google Scholar]
- Bostom AG, Gohh RY, Beaulieu AJ, Nadeau MR, Hume AL, Jacques PF, et al. Treatment of hyperhomocysteinemia in renal transplant recipients. A randomized, placebo‐controlled trial. Annals of Internal Medicine 1997; Vol. 127, issue 12:1089‐92. [MEDLINE: ] [DOI] [PubMed]
Bostom 2000 {published data only}
- Bostom AG, Shemin D, Bagley P, Massy ZA, Zanabli A, Christopher K, et al. Controlled comparison of L‐5‐methyltetrahydrofolate versus folic acid for the treatment of hyperhomocysteinemia in hemodialysis patients. [erratum appears in Circulation 2000 Aug 1;102(5):598]. Circulation 2000;101(24):2829‐32. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Bostom AG, Shemin D, Bagley P, Massy ZA, Zanabli A, Spiegel P, et al. Reduced folates are an expensive treatment for hyperhomocysteinemia in hemodialysis patients with no greater efficacy than folic acid: results of a controlled comparison study [abstract]. Journal of the American Society of Nephrology 2000;11(Sept):257A. [CENTRAL: CN‐00550713] [Google Scholar]
- Bostom AG, Shemin D, Gohh RY, Beaulieu AJ, Bagley P, Massy ZA, et al. Treatment of hyperhomocysteinemia in hemodialysis patients and renal transplant recipients. Kidney International ‐ Supplement 2001;59(78):S246‐52. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Bostom AG, Shemin D, Gohh RY, Beaulieu AJ, Jacques PF, Dworkin L, et al. Treatment of mild hyperhomocysteinemia in renal transplant recipients versus hemodialysis patients. Transplantation 2000;69(10):2128‐31. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Jurewicz 2003 {published data only}
- Jurewicz WA. Tacrolimus versus cyclosporin immunosuppression: long‐term outcome in renal transplantation. Nephrology Dialysis Transplantation 2003 May;18 Suppl 1:i7‐11. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Juskowa 2006 {published data only}
- Juskowa J, Lewandowska M, Bartlomiejczyk I, Foroncewicz B, Korabiewska I, Niewczas M, et al. Physical rehabilitation and risk of atherosclerosis after successful kidney transplantation. Transplantation Proceedings 2006;38(1):157‐60. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
LANDMARK 2 Study 2009 {published data only}
- Kaisar M, Armstrong K, Prins J, Marwick T, Johnson D, Hawley C, et al. The impact of aggressive cardiovascular risk modification on carotid intima media thickness and brachial artery reactivity in renal transplant recipients [abstract no: 132]. Nephrology 2008;13(Suppl 3):A134. [CENTRAL: CN‐00766731] [Google Scholar]
- Kaisar MO, Armstrong K, Hawley C, Campbell S, Mudge D, Johnson DW, et al. Adiponectin is associated with cardiovascular disease in male renal transplant recipients: baseline results from the LANDMARK 2 study. BMC Nephrology 2009;10:29. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lash 1998 {published data only}
- Lash JP, Cardoso LR, Mesler PM, Walczak DA, Pollak R. The effect of garlic on hypercholesterolemia in renal transplant patients. Transplantation Proceedings 1998;30(1):189‐91. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Manrique 2005 {published data only}
- Manrique J, Diaz A, Gavira JJ, Hernandez A, Pujante D, Errasti P. Preliminary results of the effect of treatment of hyperhomocysteinemia and its relationship with inflammation, coagulation status, and endothelial function after renal transplantation. Transplantation Proceedings 2005;37(9):3782‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Manrique J, Errasti P, Orbe J, Paramo JA, Rodriguez JA. Folic acid and B vitamins improve hyperhomocysteinemia‐induced cardiovascular risk profile in renal transplant recipients. Journal of Thrombosis & Haemostasis 2007;5(5):1072‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Marcucci 2002 {published data only}
- Bertoni E, Rosati A, Zanazzi M, Marcucci R, Fedi S, Abbate R, et al. Vitamin supplement reduces the progression of atherosclerosis in hyperhomocysteinemic renal transplant recipients [abstract no: F‐FC055]. Journal of the American Society of Nephrology 2002;13(September, Program & Abstracts):74P. [CENTRAL: CN‐00444418] [Google Scholar]
- Marcucci R, Bertoni E, Zanazzi M, Rosati A, Fedi S, Rogolino A, et al. Vitamin supplementation reduces the progression of atherosclerosis in hyperhomocysteinemic renal transplant recipients [abstract]. Nephrology Dialysis Transplantation 2002;17(Suppl 11):31. [CENTRAL: CN‐00520363] [DOI] [PubMed] [Google Scholar]
- Marcucci R, Zanazzi M, Bertoni E, Rosati A, Fedi S, Lenti M, et al. Homocysteine‐lowering therapy and carotid intima‐media thickness in renal transplant recipients. Transplantation Proceedings 2005;37(6):2491‐2. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Marcucci R, Zanazzi M, Bertoni E, Rosati A, Fedi S, Lenti M, et al. Vitamin supplementation reduces the progression of atherosclerosis in hyperhomocysteinemic renal‐transplant recipients. Transplantation 2003;75(9):1551‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Rosati A, Bertoni E, Zanazzi M, Marcucci R, Fedi S, Castellani S, et al. Vitamin supplement reduces the progression of atherosclerosis in hyperhomocysteinemic renal transplant recipients [abstract]. Nephrology Dialysis Transplantation 2003;18(Suppl 4):510. [CENTRAL: CN‐00447462] [DOI] [PubMed] [Google Scholar]
Nafar 2009 {published data only}
- Nafar M, Khatami F, Kardavani B, Farjad R, Pour‐Reza‐Gholi F, Firouzan A, et al. Role of folic acid in atherosclerosis after kidney transplant: a double‐blind, randomized, placebo‐controlled clinical trial. Experimental & Clinical Transplantation 2009;7(1):33‐9. [MEDLINE: ] [PubMed] [Google Scholar]
Perez 2004 {published data only}
- Perez L, Sanchez E, Hernandez D, Vega MJ, Alvarez A, Delgado P, et al. Vitamin supplements improve the lipid profile in renal transplant patients: a randomized clinical trial [abstract no: SU‐PO994]. Journal of the American Society of Nephrology 2004;15(Oct):747A. [CENTRAL: CN‐00677746] [Google Scholar]
Rymarz 2009 {published data only}
- Rymarz A, Durlik M, Rydzewski A. Intravenous administration of N‐acetylcysteine reduces plasma total homocysteine levels in renal transplant recipients. Annals of Transplantation 2009;14(4):5‐9. [MEDLINE: ] [PubMed] [Google Scholar]
Savaj 2002 {published data only}
- Savaj S, Rezakhani S, Porooshani F, Ghods AJ. Effect of folic acid therapy on serum homocysteine level in renal transplant recipients. Transplantation Proceedings 2002;34(6):2419. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Shemin 2001 {published data only}
- Shemin D, Bostom AG, Selhub J. Treatment of hyperhomocysteinemia in end‐stage renal disease. American Journal of Kidney Diseases 2001;38(4 Suppl 1):S91‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Teplan 2003b {published data only}
- Teplan V, Schuck O, Hyanek J, Poledne R, Vitko S. Effective treatment of hyperhomocysteinemia and obesity reduces the risk after renal transplantation [abstract no: T711]. Nephrology Dialysis Transplantation 2003;18(Suppl 4):509. [CENTRAL: CN‐00447972] [Google Scholar]
Xu 2005a {published data only}
- Xu T, Wang XF, Qu XK, Ye HY, Huang XB, Zhang XP, et al. Treatment of hyperhomocysteinemia and endothelial dysfunction in renal‐transplant recipients with vitamin B. Chung‐Hua Wai Ko Tsa Chih [Chinese Journal of Surgery] 2005; Vol. 43, issue 14:940‐3. [MEDLINE: ] [PubMed]
- Xu T, Zhang XW, Qu XK, Ye HY, Huang XB, Zhang XP, et al. Treatment of hyperhomocysteinemia and endothelial dysfunction in renal transplant recipients with B vitamins in the Chinese population. Journal of Urology 2008;179(3):1190‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Additional references
Albert 2008
- Albert CM, Cook NR, Gaziano JM, Zaharris E, MacFadyen J, Danielson E, et al. Effect of folic acid and B vitamins on risk of cardiovascular events and total mortality among women at high risk for cardiovascular disease: a randomized trial. JAMA 2008;299(17):2027‐36. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
ALERT Study 2003
- Holdaas H, Fellstrom B, Jardine AG, Holme I, Nyberg G, Fauchald P, et al. Effect of fluvastatin on cardiac outcomes in renal transplant recipients: a multicentre, randomised, placebo‐controlled trial. Lancet 2003;361(9374):2024‐31. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bonaa 2006
- Bonaa KH, Njolstad I, Ueland PM, Schirmer H, Tverdal A, Steigen T, et al. Homocysteine lowering and cardiovascular events after acute myocardial infarction. New England Journal of Medicine 2006;354(15):1578‐88. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
CARI 2012
- CARI. Care for kidney transplant recipients. 2012. www.cari.org.au/Transplantation/transplantation%20care%20of%20recipients/transplant_care_of_recipients.html (accessed 23 March 2015).
Clarke 2010
- Clarke R, Halsey J, Lewington S, Lonn E, Armitage J, Manson JE, et al. Effects of lowering homocysteine levels with B vitamins on cardiovascular disease, cancer, and cause‐specific mortality: Meta‐analysis of 8 randomized trials involving 37 485 individuals. Archives of Internal Medicine 2010;170(18):1622‐31. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Crider 2011
- Crider KS, Bailey LB, Berry RJ. Folic acid food fortification‐its history, effect, concerns, and future directions. Nutrients 2011;3(3):370‐84. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
D'Angelo 1997
- D'Angelo A, Selhub J. Homocysteine and thrombotic disease. Blood 1997;90(1):1‐11. [MEDLINE: ] [PubMed] [Google Scholar]
Ducloux 2000
- Ducloux D, Motte G, Challier B, Gibey R, Chalopin JM. Serum total homocysteine and cardiovascular disease occurrence in chronic, stable renal transplant recipients: a prospective study. Journal of the American Society of Nephrology 2000;11(1):134‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
EBPG 2002
- EBPG Expert Group on Renal Transplantation. European best practice guidelines for renal transplantation. Section IV: Long‐term management of the transplant recipient. IV.5.5. Cardiovascular risks. Hyperhomocysteinaemia. Nephrology Dialysis Transplantation 2002;17 Suppl 4:28‐9. [MEDLINE: ] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315(7109):629‐34. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gallagher 2009
- Gallagher M, Jardine M, Perkovic V, Cass A, McDonald S, Petrie J, et al. Cyclosporine withdrawal improves long‐term graft survival in renal transplantation. Transplantation 2009;87(12):1877‐83. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
HSC 2002
- Homocysteine Studies Collaboration. Homocysteine and risk of ischemic heart disease and stroke: a meta‐analysis. JAMA 2002;288(16):2015‐22. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hubner 2007
- Hubner RA, Houlston RD, Muir KR. Should folic acid fortification be mandatory? No. BMJ 2007;334(7606):1253. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jamison 2007
- Jamison RL, Hartigan P, Kaufman JS, Goldfarb DS, Warren SR, Guarino PD, et al. Effect of homocysteine lowering on mortality and vascular disease in advanced chronic kidney disease and end‐stage renal disease: a randomized controlled trial. JAMA 2007;298(10):1163‐70. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Jardine 2012
- Jardine MJ, Kang A, Zoungas S, Navaneethan SD, Ninomiya T, Nigwekar SU, et al. The effect of folic acid based homocysteine lowering on cardiovascular events in people with kidney disease: systematic review and meta‐analysis. BMJ 2012;344:e3533. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kasiske 1996
- Kasiske BL, Guijarro C, Massy ZA, Wiederkehr MR, Ma JZ. Cardiovascular disease after renal transplantation. Journal of the American Society of Nephrology 1996;7(1):158‐65. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
KDIGO 2009
- Kidney Disease: Improving Global Outcomes (KDIGO)Transplant Work Group. KDIGO clinical practice guideline for the care of kidney transplant recipients. American Journal of Transplantation 2009;9 Suppl 3:S1‐157. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lang 2000
- Lang D, Kredan MB, Moat SJ, Hussain SA, Powell CA, Bellamy MF, et al. Homocysteine‐induced inhibition of endothelium‐dependent relaxation in rabbit aorta: role for superoxide anions. Arteriosclerosis, Thrombosis, & Vascular Biology 2000;20(2):422‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lentz 1991
- Lentz SR, Sadler JE. Inhibition of thrombomodulin surface expression and protein C activation by the thrombogenic agent homocysteine. Journal of Clinical Investigation 1991;88(6):1906‐14. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lentz 1996
- Lentz SR, Sobey CG, Piegors DJ, Bhopatkar MY, Faraci FM, Malinow MR, et al. Vascular dysfunction in monkeys with diet‐induced hyperhomocyst(e)inemia. Journal of Clinical Investigation 1996;98(1):24‐9. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lonn 2006
- Lonn E, Yusuf S, Arnold MJ, Sheridan P, Pogue J, Micks M, et al. Homocysteine lowering with folic acid and B vitamins in vascular disease. New England Journal of Medicine 2006;354(15):1567‐77. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Massy 1994
- Massy ZA, Chadefaux‐Vekemans B, Chevalier A, Bader CA, Drüeke TB, Legendre C, et al. Hyperhomocysteinaemia: a significant risk factor for cardiovascular disease in renal transplant recipients. Nephrology Dialysis Transplantation 1994;9(8):1103‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
McCully 1996
- McCully KS. Homocysteine and vascular disease. Nature Medicine 1996;2(4):386‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
NIH 2007
- National Institutes of Health. U.S. Renal Data System, USRDS 2007 Annual Data Report: Atlas of End‐Stage Renal Disease in the United States. 2007. www.usrds.org/atlas07.aspx (accessed 23 March 2015).
Orioli 2011
- Orioli IM, Lima do Nascimento R, Lopez‐Camelo JS, Castilla EE. Effects of folic acid fortification on spina bifida prevalence In Brazil. Birth Defects Research 2011;91(9):831‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Schnyder 2002
- Schnyder G, Roffi M, Flammer Y, Pin R, Hess OM. Effect of homocysteine‐lowering therapy with folic acid, vitamin B12, and vitamin B6 on clinical outcome after percutaneous coronary intervention: the Swiss Heart study: a randomized controlled trial. JAMA 2002;288(8):973‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Starkebaum 1986
- Starkebaum G, Harlan JM. Endothelial cell injury due to copper‐catalyzed hydrogen peroxide generation from homocysteine. Journal of Clinical Investigation 1986;77(4):1370‐6. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
The Renal Association 2010
- Holt S, Goldsmith D. Cardiovascular disease in CKD. 2010. www.renal.org/guidelines/modules/cardiovascular‐disease‐in‐ckd#Summary2 (accessed 23 March 2015).
Toole 2004
- Toole JF, Malinow MR, Chambless LE, Spence JD, Pettigrew LC, Howard VJ, et al. Lowering homocysteine in patients with ischemic stroke to prevent recurrent stroke, myocardial infarction, and death: the Vitamin Intervention for Stroke Prevention (VISP) randomized controlled trial. JAMA 2004;291(5):565‐75. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Vianna 2007
- Vianna AC, Mocelin AJ, Matsuo T, Morais‐Filho D, Largura A, Delfino VA, et al. Uremic hyperhomocysteinemia: a randomized trial of folate treatment for the prevention of cardiovascular events. Hemodialysis International 2007;11(2):210‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Vollset 2013
- Vollset SE, Clarke R, Lewington S, Ebbing M, Halsey J, Lonn E, et al. Effects of folic acid supplementation on overall and site‐specific cancer incidence during the randomised trials: meta‐analyses of data on 50,000 individuals. Lancet 2013;381(9871):1029‐36. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wald 2002
- Wald DS, Law M, Morris JK. Homocysteine and cardiovascular disease: evidence on causality from a meta‐analysis. BMJ 2002;325(7374):1202. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Winkelmayer 2005
- Winkelmayer WC, Kramar R, Curhan GC, Chandraker A, Endler G, Födinger M, et al. Fasting plasma total homocysteine levels and mortality and allograft loss in kidney transplant recipients: a prospective study. Journal of the American Society of Nephrology 2005;16(1):255‐60. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Wrone 2004
- Wrone EM, Hornberger JM, Zehnder JL, McCann LM, Coplon NS, Fortmann SP. Randomized trial of folic acid for prevention of cardiovascular events in end‐stage renal disease. Journal of the American Society of Nephrology 2004;15(2):420‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Yap 2003
- Yap S. Classical homocystinuria: vascular risk and its prevention. Journal of Inherited Metabolic Disease 2003;26(2‐3):259‐65. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Zoungas 2006
- Zoungas S, McGrath BP, Branley P, Kerr PG, Muske C, Wolfe R, et al. Cardiovascular morbidity and mortality in the Atherosclerosis and Folic Acid Supplementation Trial (ASFAST) in chronic renal failure: a multicenter, randomized, controlled trial. Journal of the American College of Cardiology 2006;47(6):1108‐16. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Kang 2009
- Kang A, Nigwekar SU, Perkovic V, Kulshrestha S, Zoungas S, Navaneethan SD, et al. Interventions for lowering plasma homocysteine levels in kidney transplant recipients. Cochrane Database of Systematic Reviews 2009, Issue 3. [DOI: 10.1002/14651858.CD007910] [DOI] [PMC free article] [PubMed] [Google Scholar]


