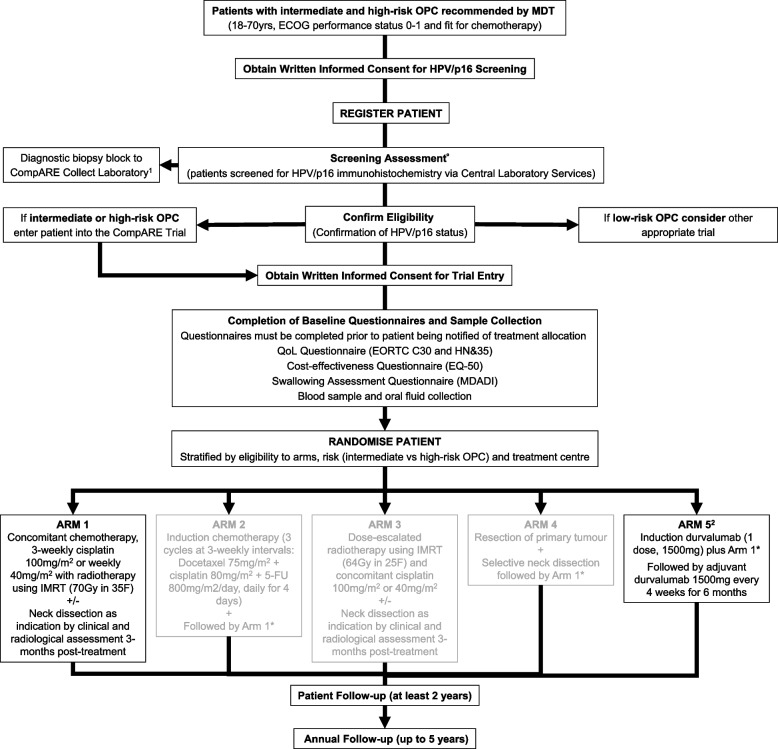
Fig. 1.
CompARE trial design. CompARE is a pragmatic phase III open-label multicenter randomised controlled trial with an adaptive multi-arm multi-stage design. Recruitment remains open for arms 1 and 5. Recruitment was suspended to arm 2 on 9 January 2017, recruitment suspended to arm 3 on 12 September 2019, and recruitment suspended to arm 4 on 7 February 2019. 1Samples collected for translational research (CompARE Collect) if the patient has consented. 2Additional baseline tests (clinical chemistry) are required for patients randomised to arm 5. *Neck dissection is required if a persistent disease is identified in the neck on clinical and radiological imaging at 3 months post-treatment. °Screening assessment: confirmation of HPV/p16 status should be provided by central laboratory services. For randomisation purposes, local p16 test results can be used; however, the diagnostic biopsy sample must still be sent to central laboratory services for confirmation. 5-FU, 5-fluorouracil; ECOG, Eastern Cooperative Oncology Group; HPV, human papillomavirus; IMRT, intensity-modulated radiotherapy; MDT, multidisciplinary team; OPC, oropharyngeal cancer