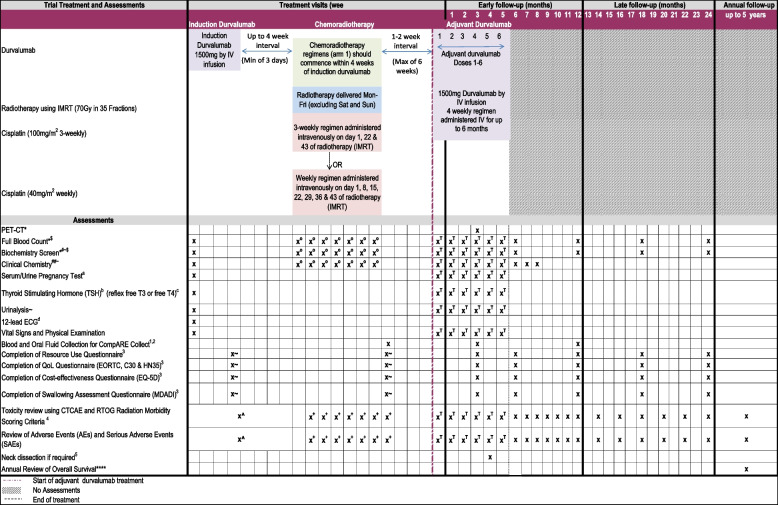
Fig. 3.
Schedule of events for arm 5 of the CompARE trial. Schedule of events for treatment arm 5, induction durvalumab plus arm 1 followed by adjuvant durvalumab. *Assessment part of standard practice. ****Patient followed up annually for survival data for up to 5 years. #Biochemistry screen: alkaline phosphatase (ALP), alanine transferase (ALT), biocarbonate, calcium, creatinine, glomerular filtration rate, liver function tests (LFTs), glucose, magnesium, potassium, sodium, total bilirubin, total protein, urea, or blood urea nitrogen. Serum or plasma analysis will include albumin, glucose, and gamma-glutamyl transferase. Biochemistry screen must take place within 120 h prior to durvalumab infusion. ##Clinical chemistry screen: amylase, lactose dehydrogenase, and aspartate aminotransferase (AST). It is preferable that both amylase and lipase parameters are assessed. For sites where only one of these parameters is routinely measured then either lipase or amylase is acceptable. Clinical chemistry screen must take place within 120 h prior to durvalumab infusion. $Results for LFTs, electrolytes, full blood count, and creatinine must be available before commencing an infusion (within 120 h) and reviewed by the treating physician or investigator prior to dosing. ~Tests for ALT, AST, ALP, and total bilirubin must be conducted and assessed concurrently. If total bilirubin is ≥ 2× upper limit of normal (and no evidence of Gilbert’s syndrome) then fractionate into direct and indirect bilirubin. aFor women of childbearing potential only. A urine or serum pregnancy test is acceptable. Women of childbearing potential are required to have a pregnancy test within 7 days prior to the first dose of study drug. bIf thyroid-stimulating hormone (TSH) is measured within 14 days prior to day 1 (first durvalumab infusion day), it does not need to be repeated at day 1. cFree T3 or free T4 will only be measured if TSH is abnormal or if there is clinical suspicion of an AE related to the endocrine system. dAny clinically significant abnormalities detected require triplicate ECG results. oCisplatin 100 mg/m2 3-weekly: full blood count, biochemistry screen, and clinical chemistry screen to be performed 3-weekly. Cisplatin 40 mg/m2 weekly: full blood count, biochemistry screen, and clinical chemistry screen to be performed weekly. +Toxicity and adverse events assessed during chemoradiotherapy. TAssessments to be performed 4-weekly during adjuvant durvalumab treatment. ^Toxicity and adverse events assessed 2-weekly. 1Samples collected if the patient has consented for CompARE Collect. 2Blood and oral fluid samples should be collected at recurrence or progression (formalin-fixed paraffin-embedded tissue block or needle aspirate sample should also be collected if recurrence is confirmed by histology/cytology). 3Questionnaires to be completed by the patient in the clinic at defined visits. 4Toxicity will be reviewed using CTCAE version 4.0 and version 3.0 for scoring mucositis. The RTOG Radiation Morbidity Scoring Criteria will be used to grade late side effects due to radiotherapy. 5Neck dissection is required if persistent disease is identified in the neck on imaging (PET-CT or contrast CT or contrast MRI) at 3 months post-chemoradiotherapy treatment. The same modality PET CT or CT or MRI should be used for all arms. ~Questionnaires to be completed at the end of chemoradiotherapy and end of durvalumab treatment