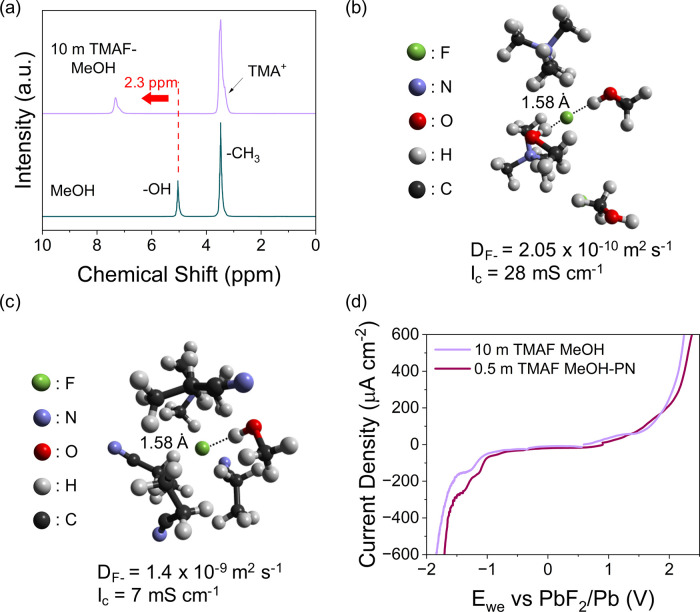
Figure 3.
Electrolyte characterization. (a) 1H NMR showing a shift to higher frequencies in the proton peak of the -OH group in 10 m TMAF in MeOH compared to pure MeOH. (b and c) Snapshot of Monte Carlo simulation boxes showing F– solvated by (b) methanol molecules in the 10 m solution and (c) methanol and propionitrile molecules in the 0.5 TMAF-MeOH-PN ternary solution. Their respective diffusion coefficients (DF-) and ionic conductivities (Ic) are also reported. In both electrolytes the distance between fluoride ions and the proton of MeOH is 1.58 Å. (d) Electrochemical stability window of 10 m TMAF in MeOH and 0.5 m TMAF in MeOH-PN measured at a scan rate of 1 mV s–1.