Figure 2.
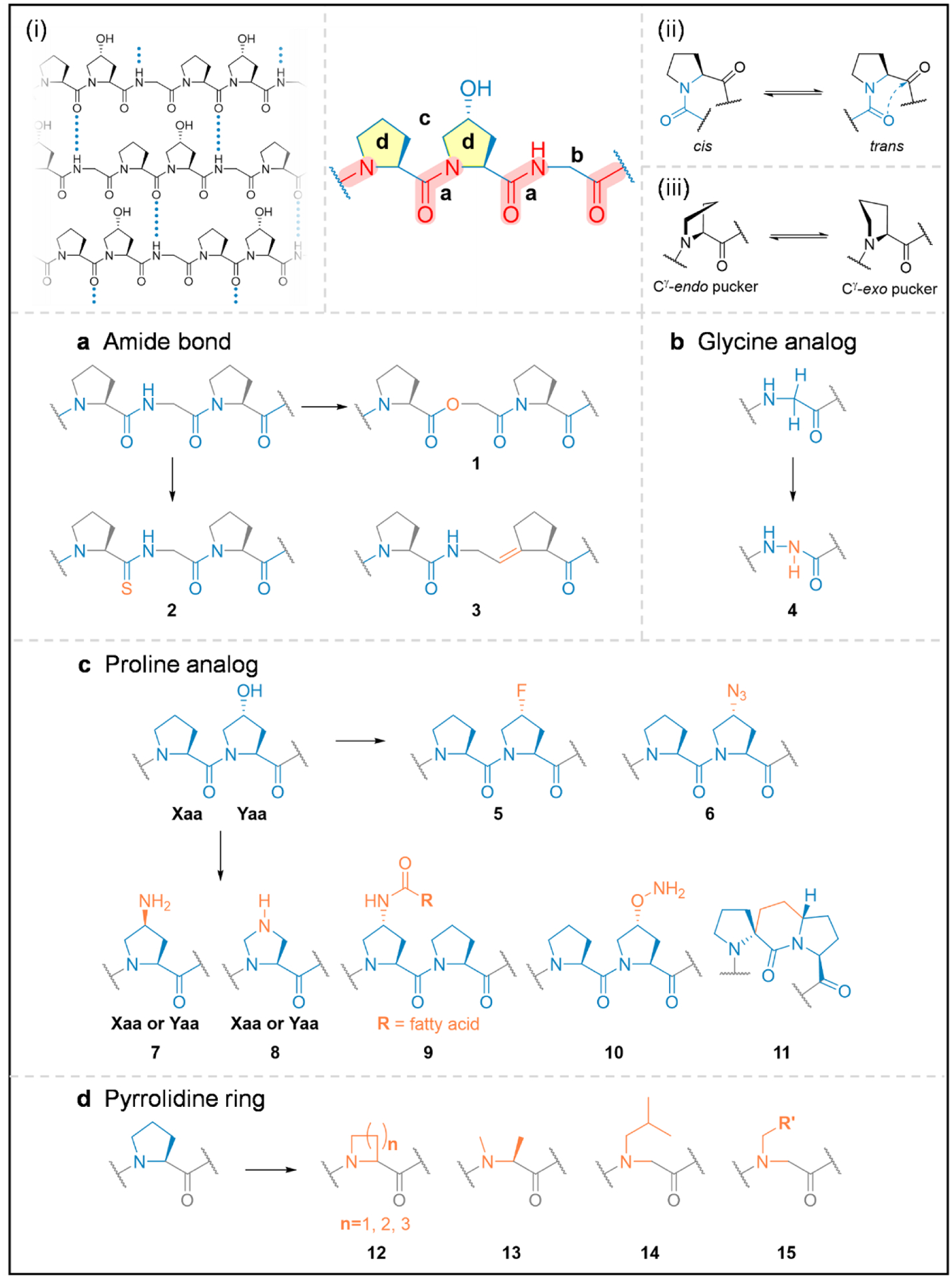
The structural model of the collagen hybridizing peptides, whose triple-helix folding is governed by the interstrand N-H(Gly)…O=C hydrogen bonding (i), the trans/cis conformation of the Pro-amide bond (ii), and the pucker of the pyrrolidine ring (iii). To gain insights into the peptide’s propensity to form the collagen triple-helix, extensive chemical modifications (in orange) have been made to its various structural sub-units (blue) including the amide bond (a), the Gly and Hyp residues (b,c), as well as the pyrrolidine ring (d).
