Abstract
Objective
Virtual care (VC) is an accepted modality of care delivery, and shared decision‐making (SDM) benefits patients with rheumatologic and chronic conditions (RCCs). Unfortunately, research suggests reduced quality of SDM during VC. This study explores the benefits and shortcomings of SDM regarding RCCs during VC with suggestions for optimally using VC during SDM.
Methods
Following Stiggelbout's framework for SDM, we conducted focus groups of patients with RCCs and providers to understand their experiences with SDM during VC, probing for facilitating and challenging factors. We conducted content analysis of the transcripts, defining themes, and inductively reasoned to identify relationships among themes. We summarized the facilitators, barriers, and opportunities for improving SDM during VC that participants proposed.
Results
Virtual SDM shares several similarities with in‐person practice, as both draw upon trusting patient‐provider relationships, following the same general steps, and relying on effective communication. VC presents solutions for known barriers to in‐person SDM, expanding time for making decisions and access to care. Technology and virtual health systems introduce new barriers to SDM, and participants list opportunities for overcoming these concerns.
Conclusion
VC is a tool that can enhance and even support superior SDM compared with in‐person visits when implemented successfully, a condition requiring the development of nuanced skills to correctly identify when and how to best use VC for SDM as well as technology and health care structures that integrate SDM into VC. Therefore, patients, providers, insurance carriers, and policy makers all contribute to the success of SDM among RCCs during VC.
INTRODUCTION
Shared decision‐making (SDM), essential to managing rheumatologic and chronic conditions (RCCs), occurs when patients and providers work together to choose options that align with patients’ preferences. 1 Stiggelbout outlines four steps for SDM whereby patients and providers announce decisions to be made, list options, incorporate patient preferences, and select plans. 1 Successful SDM can enhance patient knowledge, adherence, and satisfaction while optimizing health equity 2 , 3 , 4 , 5 and improving outcomes.
SIGNIFICANCE & INNOVATIONS.
Shared decision‐making (SDM) is essential to the care of patients with rheumatologic and chronic conditions (RCCs).
Virtual care (VC) expands access to patients with RCCs, yet studies suggest SDM is reduced during VC.
VC can enhance SDM by giving patients with RCCs more time to make choices and providing alternate avenues for patient education.
An essential component of VC, SDM occurs successfully when VC skills, such as communication, interpersonal relationship building, and constructing health systems, support the steps of SDM during virtual encounters and when patients and providers can implement these skills virtually.
Despite these benefits, providers implement SDM inconsistently, 6 and patients who identify with underrepresented groups report lower quality SDM, contributing to health disparities. 7 Studies name time constraints, providers’ attitudes, and patient health literacy as limitations, 8 prompting shareholders to overcome these barriers to achieve the benefits of SDM.
Virtual care (VC) takes place whenever technology facilitates care delivery. 9 VC sometimes can promote SDM; for example, surgery patients reported equivalent quality of SDM during VC versus in‐person visits. 10 Unfortunately, other accounts demonstrate challenges. Patients with RCCs stated that they “lost their voice” when VC emerged, and analyses of teleconsultations among primary care providers, specialists, and patients showed reduced patient participation. 11 , 12 This may be particularly true for underrepresented groups for whom the “digital divide” creates differential access to technology, compounding disparities already observed in SDM. 13
With widespread integration of VC, patients and providers are tasked with optimizing SDM through in‐person and virtual methods. In this study, we apply Stiggelbout's model as a framework for exploring the facilitators and barriers to SDM during VC among patients with RCCs. 1 Second, we investigate the role VC plays in SDM and strategies for optimizing SDM during VC.
MATERIALS AND METHODS
Research team
We assembled a research team of physicians (LZ, CM) and people with RCCs (ENT, TWR) with expertise in moderating focus groups, VC, and SDM, as well as qualitative research (EAB, JLH).
Study participants
In December 2021, we recruited patients and providers through advertisements posted on Washington University in St. Louis School of Medicine's (WUSM) Volunteer for Health Study social media and the International Foundation for Autoimmune and Autoinflammatory Arthritis’ (AiArthritis) podcasts and webpage. We invited additional providers from the VC literature through email.
Advertisements linked to an online survey that gathered demographic information, including experience with VC, role as patient or provider, and patients’ diagnoses. From the survey results, we selected participants, sampling to maximize diversity primarily from underrepresented groups (defined as racial, ethnic, and sex, and gender minorities), secondarily from geographic location, and lastly for primary diagnosis or medical specialty. We focused on rheumatic diseases while including other chronic conditions to enhance the transferability of results. The survey included an information sheet through which participants granted their consent. Demographic data were collected using REDCap tools hosted at WUSM. 14 Methods complied with the Helsinki Declaration, as approved by the WUSM Institutional Review Board (#202109117).
Focus groups
We organized focus groups to maximize idea generation among participants for topics regarding SDM during VC. Two‐to‐five patients and two providers participated in each focus group together because this dynamic generated the most discussion of opportunities to optimize SDM during a pilot. A research physician (LZ or CM) and patient (ENT or TWR) conducted the semistructured interviews. Five focus groups, each lasting about 90 minutes, occurred from February to June 2022 and were conducted as videoconferences, beginning with verbal informed consent before recording and transcribing the interviews. During data analysis meetings, we discussed whether there were new insights or unanswered questions. We continued hosting focus groups until no new information emerged (saturation). Participants received $30 Amazon gift cards.
We wrote an interview guide (Supplement 1) that explored the benefits, challenges, and opportunities for SDM during VC following Stiggelbout's model (1). The guide prompted participants to reflect on examples implementing the steps of SDM. The interview concluded with discussions of how technology affects SDM and opportunities to overcome barriers.
Qualitative analysis
The research team performed conventional content analysis of transcripts while simultaneously leading subsequent focus groups, providing opportunities to pursue emerging concepts in interviews and determine data saturation. 15 JLH oversaw the analysis. LZ, CM, ENT, and TWR reviewed the transcripts from the first two focus groups, generating the codebook. Then, LZ and TWR applied the codebook to transcripts from the third and fourth focus groups, while CM and ENT did the same for the fifth focus group. The team regrouped after the final focus group, refined codes, and identified themes. Then, LZ and TWR verified the coding for the transcripts from the second and fifth focus groups, while CM and ENT did the same for the first, third, and fourth focus groups. Through this system, researchers coded transcripts iteratively to refine the codebook, reviewed all transcripts to understand the data, and verified other team members’ coding. The team discussed disagreements and revised coding until reaching consensus.
The team used inductive reasoning to synthesize themes and the interactions among them into a theory. From the transcripts, we collected facilitators and barriers to SDM during VC and examples for optimizing SDM, supplementing suggestions that matched the interviews’ context when participants did not provide explicit recommendations. We used HyperRESEARCH (Researchware, Inc) for analysis and the Consolidated Criteria for Reporting Qualitative Research (COREQ) checklist for manuscript preparation (Supplement 2). 16
Member checking and educational needs assessment
To enhance the trustworthiness of findings, in May 2023, we emailed participants inviting them to review and comment on our initial findings for accuracy in an online REDCap survey. The research team reviewed and applied the submitted comments. In the survey, participants also rated the importance of SDM to VC and the need for initiatives teaching SDM during VC. Participants received a $30 Amazon gift card for completing the survey.
RESULTS
Demographics
Twenty‐six people participated in focus groups; the majority were female (55.7%) and patients (65.4%) (Table 1). Half (50%) identified with an underrepresented group. Participants represented five geographic regions of the United States and one international location. Most patients reported having inflammatory arthritis (88.2%), and the majority of providers specialized in rheumatology (66.7%).
Table 1.
Characteristics of focus group participants, organized by patients and health care providers*
| Patient characteristics (N = 17) | Number (% of patient participants) |
|---|---|
| Gender | |
| Male | 5 (29.4) |
| Female | 11 (64.7) |
| Nonbinary/third gender | 1 (5.9) |
| Race and ethnicity | |
| Black or African American | 2 (11.8) |
| Hispanic or LatinX | 2 (11.8) |
| White or Caucasian | 10 (58.8) |
| Multiracial or biracial | 3 (17.6) |
| Geographic location | |
| Northeast United States | 3 (17.6) |
| Southeast United States | 1 (5.9) |
| Midwest United States | 7 (41.2) |
| Pacific United States | 5 (29.4) |
| International | 1 (5.9) |
| Diagnosesa | |
| Chronic conditions | |
| Asthma | 4 (23.5) |
| Cardiovascular disease | 7 (41.2) |
| Chronic infections b | 1 (5.9) |
| Chronic kidney disease | 2 (11.8) |
| Chronic pain syndrome c | 8 (47.1) |
| Endocrine disorder d | 6 (35.3) |
| Rheumatologic conditions | |
| Autoimmune connective tissue disease e | 4 (23.5) |
| Inflammatory arthritis f | 15 (88.2) |
| Osteoarthritis | 8 (47.1) |
| Regional musculoskeletal syndrome g | 3 (17.6) |
| Type of virtual care | |
| Telephone | 1 (5.9) |
| Video | 3 (17.6) |
| Telephone and video | 13 (76.5) |
| Use of virtual care | |
| 1–2 times total | 3 (17.6) |
| 3–5 times total | 4 (23.5) |
| 6 or more times | 10 (58.8) |
| Health care provider characteristics (N = 9) | Number (% of provider participants) |
|---|---|
| Sex | |
| Male | 5 (55.6) |
| Female | 4 (44.4) |
| Race and ethnicity | |
| Asian or Pacific Islander | 1 (11.1) |
| Black or African American | 3 (33.3) |
| Hispanic or LatinX | 1 (11.1) |
| Native American or Alaskan | 1 (11.1) |
| White or Caucasian | |
| Geographic location | |
| Northeast United States | 3 (33) |
| Southeast United States | 1 (11.1) |
| Midwest United States | 2 (22.2) |
| Pacific United States | 2 (22.2) |
| Noncontiguous United States | 1 (11.1) |
| Profession | |
| Physician | 7 (77.7) |
| Physician assistant | 1 (11.1) |
| Nurse practitioner | 1 (11.1) |
| Specialty | |
| Dermatology | 1 (11.1) |
| Hematology | 1 (11.1) |
| Infectious diseases | 1 (11.1) |
| Rheumatology | 6 (66.7) |
| Type of practice | |
| Academic | 6 (66.7) |
| Private practice | 1 (11.1) |
| Nonprofit public health | 1 (11.1) |
| Veterans Affairs | 1 (11.1) |
| Type of virtual care | |
| Telephone and video | 9 (100) |
| Use of virtual care | |
| 1 time a week | 3 (33.3) |
| 2–5 times a week | 3 (33.3) |
| 6 or more times a week | 3 (33.3) |
aPatients were allowed to list more than one medical condition in their health history.
Chronic infections include HIV and viral hepatitis.
Chronic pain syndromes include chronic headaches and fibromyalgia.
Endocrine disorders include diabetes and thyroid disorders.
Autoimmune connective tissue diseases include antiphospholipid syndrome, lupus, and Sjögren syndrome.
Inflammatory arthritis includes ankylosing spondylitis, gout, juvenile idiopathic arthritis, and rheumatoid arthritis.
Regional musculoskeletal syndromes include carpal tunnel, rotator cuff tear, and hypermobility syndrome.
Member checking and educational needs assessment
Nineteen (73.1%) participants reviewed the results. On a three‐point scale (agree, somewhat agree, disagree), 17 participants (89.5%) agreed that the theory reflected their experience with SDM during VC, and 2 responded “somewhat.” The research team integrated suggestions, modifying our description of verbal communication as a barrier to virtual SDM.
On a four‐point scale (strongly disagree, disagree, agree, strongly agree), all (100%) agreed or strongly agreed that SDM is an essential part of the VC experience. Fifteen (78.9%) agreed or strongly agreed that differences exist between conducting SDM in person or virtually; 17 (89.5%) agreed or strongly agreed that providers should receive training on SDM during VC.
Thematic analysis and theory
We identified seven themes describing factors influencing SDM during VC, the interactions among them (Figure 1), and a list of facilitators, barriers, and strategies for optimization (Table 2). The Patient‐Provider Relationship emerged as the foundational theme for SDM during VC and includes establishing relationships, showing respect, and using awareness of patient preferences to individualize SDM. The process of Shared Decision‐Making became a central theme, whereby patients and providers follow the same general steps for SDM during in person or VC, though successful virtual implementation requires nuanced skills and factors highlighted in other themes. The themes of Communicating (a bidirectional exchange between patients and providers that uses verbal and nonverbal cues), Sharing Information (conveying data in the form of virtual histories, physical examinations, and educational resources while checking for understanding), and Choosing (providing patients time and autonomy to make decisions with follow‐up afterward) are skills that influence the success of SDM during VC. Finally, Technology and the Health Care System (eg, medical systems, insurance coverage, and health policies particular to VC) emerged as external factors that can affect SDM during VC. Collectively, these themes shape patient care scenarios and dictate whether SDM is best performed virtually or in person. We provide supporting quotes, minimally edited for readability, and attribute them to participant role.
Figure 1.
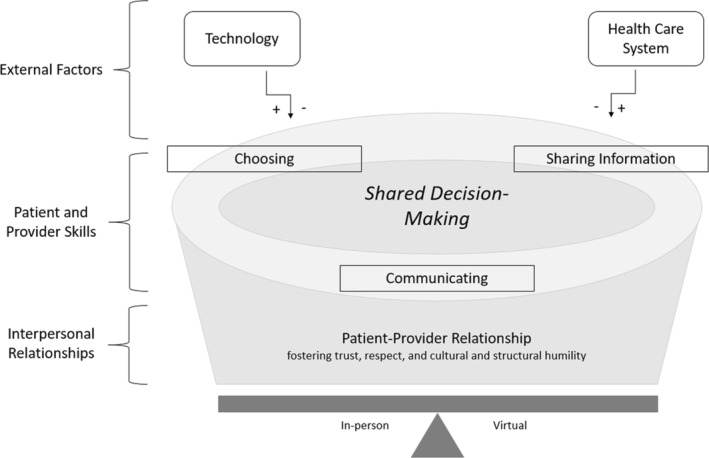
SDM during virtual care. Trusting patient‐provider relationships form the foundation for SDM, both in person and virtually, and patients and providers draw upon this relationship to support the SDM process. Whether in person or virtual, providers and patients generally follow the following four steps for SDM: (1) Stating a decision needs to be made, (2) listing options, (3) incorporating patient preferences and answering patient questions, and (4) making a decision. The three skills of communicating, sharing information, and choosing influence the success of SDM during virtual care. Technology and the health care system are external factors that can positively or negatively affect SDM during virtual care. Collectively, these factors determine if SDM is better achieved in person or virtually. SDM, shared decision‐making.
Table 2.
List of facilitators, barriers, and strategies to optimize SDM during VC collated from focus group transcripts with supplements from the research team*
| Facilitators | Barriers | Strategies for optimization |
|---|---|---|
| Most decisions can be made virtually following the same general SDM steps used in person | Emotionally charged decisions, new diagnoses, and severe or end‐stage conditions are better suited for in‐person SDM | Anticipate visits requiring in‐person SDMa |
| Trusting patient‐provider relationship; active listening; maximized eye contact via camera | Distrusting and dismissive interactions; reduced nonverbal communication during VC | Establish relationship in person; enhance listening by accommodating for transmission delays in VC platform; set up provider's VC workstation to maximize eye contact with patient |
| Unencumbered verbal communication: reading lips is easier during VC than when wearing masks in person | Challenged verbal communication (eg, language barrier, hard of hearing); reduced nonverbal communication; omitted clinical environmental cues (eg, condition of waiting room, friendliness of front desk staff) | Recommend VC to people with strong verbal communication or lip‐reading skills;a involve interpreters in VC encounters |
| Including consultants in VC visits; sharing documentation through the electronic health record | Consulting providers do not coordinate visits; health care billing does not allow for simultaneous consultations | Support workflows and reimbursement structures that incentivize providers to attend the same VC encounter |
| Telepresenters and care partners conduct physical examination or angle the camera optimally, sharing photographs of examination findings | Importance of tactile physical examination findings; lighting and camera angle can be difficult to achieve; limitations to virtual physical examination imposed by condition | Establish baseline examination in‐person; anticipate visits requiring in‐person examinations, such as acute worsening of condition or requisite hands‐on physical examination |
| All clinical data available virtually | Procedural, laboratory, or imaging studies that are obtained in‐person are needed | Collect clinical data before VC encounter |
| Patient education via chat, screen share, electronic education handouts, educational videos, after visit summaries | Patient education resources used in person are not available virtually (eg, virtual white board, decision‐making aids) | Adapt and validate in‐person patient education resources to the virtual platform |
| Multisite encounters; inclusion of care partners in SDM during VC | Patient environment lacks privacy | Verify patient's access to private location and willingness to incorporate care partners during VCa |
| Patient environment without distractions | Patient environment with distractions | Schedule VC encounters at times and locations of minimal distractiona |
| Competence working technology | Incompetence working technology | Provide technology support for patients |
| High quality video and audio | Poor quality video and audio | Test video and audio during the VC registration process |
| Strong, secure internet and telecommunication connections | Unstable, insecure, or no access to internet or telecommunications | Implement policies that provide internet and telecommunications access to patients;a verify patient call‐back number for use if connection is dropped |
| Enhanced access to care despite acute illness, travel, child care needs, or work obligations | Insurance does not cover VC; policies require in‐person evaluation for controlled substances; state medical licensure restrictions | Support policies granting providers national rather than state‐specific licensurea |
| Time‐saving VC workflows (eg, reduced rooming time, providers self‐scheduling VC encounters) | Limited time with patients | Use VC to check in with patients’ decision‐making |
aStrategy supplemented by research team.
SDM, shared decision‐making; VC, virtual care.
Patient‐provider relationship
Trusting relationships form the foundation for SDM. Patients trust providers when they respect patients’ identities, listen, and accept patients’ decisions. Individuals who identify with underrepresented groups especially value these actions, and providers who practice cultural humility are regarded highly:
“It's not just language, it's a cultural barrier…[People who identify as Hispanic] usually say ‘Everything hurts.’ [My mother and I] do… televisits, and my mother will say, ‘Everything hurts.” I have to be the one making sure that we explain it to the doctor.” (patient)
“I'm sorry that's happened to you. I think that's our job as physicians…to figure it out.” (provider)
Providers trust the accuracy of patient‐provided clinical information when they “know [patients] very well,” are familiar with patients’ disease course, and recognize that patients “have the same sense” of whether their condition “is controlled or not…” (provider).
Patients and providers agree that trusting relationships are best developed in person. Trusting relationships “can't just be achieved through virtual visits” (patient) because “there is something lost… with…the virtual visit…” (provider). The time needed to connect is unique to each patient and provider, and established, trusting patient‐provider relationships can transfer to VC. “Once the trust is there, [people] can share [their]…insights…during in‐person and virtual visits” (patient).
Within patient‐provider relationships, providers learn patients’ tendencies so that they can individualize SDM, presenting options patients are likely to consider and providing patients their preferred degree of autonomy. Familiarity also helps identify which decisions patients are amenable to making during VC. Emotionally charged decisions are best held in person, though what qualifies as “emotional” varies for each patient based on prior experiences and preferences:
“If I'm being told that I have cancer, I would like to be in person ‘cause….that is very emotional…I've been through so much…from a rheumatology perspective,…it's nothing to hear that I…need…surgeries….” (patient)
Themes and subthemes regarding interpersonal relationships generated from qualitative analysis of focus group transcripts with representative quotations from patient and provider participants.
- Respect for patients and patients identifying with underrepresented groups
- “It's…all of the things about building a relationship and building trust.…One of my providers…is the only one who…will correctly gender me…, ‘How are you doing Mx [last name]?’…. I ask about his life as well….It makes it easier to trust each other about medical decision‐making and feeling like we can collaborate….” (patient)
- Establishing the relationship and individualizing shared decision‐making
- “…with patients who I know very well, it's easy to come to that conclusion, because I feel as if I have a sense of where…their arthritis …is controlled or not controlled.…But when I'm talking to new patients, because I don't know them very well,…I'm not sure that I have the same sense that they fully understand everything, even though I take the time to explain it…because there is just something lost… with…the virtual visit…you really can't get a sense of, ‘Do you understand, or… am I making sense?’ or…‘How do you feel about this?’” (provider)
SDM
Participants identify SDM as a central element of patient care and outline steps for completion similar to Stiggelbout's model whether virtual or in person.
Themes and subthemes regarding patient and provider skills generated from qualitative analysis of focus group transcripts with representative quotations for patient and provider participants.
- Shared decision making
- “…in the office or telemedicine…my doctor…lays out a lot of decisions and choices,…and together we make the decision of what the next step is gonna be….” (patient)
- “…shared decision‐making…is a process…by which we inform patients about…diagnosis…, what the treatment options are, and… get patients’ input about…their own…goals…for therapy,.…answer their questions,…and make a decision that's…appropriate… for…the patient and fits in with…medical science….” (provider)
- Communication
- Verbal communication
- “I've only been doing virtual visits…during the pandemic….They… have gone very positively for me.…We (patient and provider) both come prepared,… go down our agendas,…get everything covered, and do it fairly quickly.…That's…one of the reasons why…I have definitely decided that doing a mix of virtual and in‐person visits… is…a good way to do it….” (patient)
- Nonverbal communication
- “The times that I've had…really bad encounters and I'm like, ‘You need to come into the office’…is due to minor communication mismatch.…Someone who is hard of hearing…, and I need to see you…to make sure that we are on the same page…or where there may be a language barrier, and even though I have an interpreter on the line, I'm not quite sure that we are quite getting there….Just being able to see the person and talk to them…can give you some additional insights….” (provider)
- Sharing information
- Announcing a decision
- Virtual physical examination
- Educating
- “…my…rheumatologist… might [share] his screen during… appointments online.…He comes up with charts…to educate me appropriately….” (patient)
- Providing a recommendation
- Admitting uncertainty
- “…it's not so challenging…explaining things when the condition is obvious and flagrant…I feel quite confident in my diagnosis….The challenge…[is]…where I am not 100% certain, because the symptoms are mild or on the borderline….Due to my lack…of confidence…I'm really trying to explain a lot of information and share my concerns…but I don't want you to lose trust in me either, because I'm saying, ‘I think you may have this, I don't know for sure that you do.’…That is probably the hardest thing to do virtually if it's someone that I cannot touch, cannot see in‐person…because I'm asking you to make a commitment to a process that could be harmful…It's hard for me to…really be clear on my decision and…for you the patient to be clear on your decision as well.” (provider)
- Choosing
- Time
- “…my current rheumatologist…doesn't always expect me to make the decision immediately. There are certain things where…you can just say, ‘Okay, yeah.’ …but for…a really…big decision…we actually made that decision over a period of about two… months.…During that time I had an in‐person visit, but we also continued talking through the MyChart system….She was really…supportive…I really valued that I could reach out to her, and ask her questions through that system.” (patient)
- Following up
- “I find that…about 80 to 90% of the visits…we're able to achieve most of the…objectives of the visit…If there's a change in therapy warranted, going over the options, presenting the options to patients, providing them some electronic resources, and… then circling back with them at a later date, either in person or virtually.…We make that decision…if it's needed.” (provider)
Communicating
SDM uses conversational exchanges between patients and providers, during which participants listen as much as they deliver information. A patient shared, “When I had COVID,…I spoke with my doctor in telehealth….By him listening to what I had to say…and talking about the medications, I knew that I didn't have to go in person,” signaling that communication transfers to VC and augments care delivery.
Communication includes verbal and nonverbal cues and is most effective when environment‐, patient‐, and provider‐specific factors facilitate virtual conversation. Strong telecommunications connections support conversation, and “with a high quality video and audio connection,…[participants can]…talk about…next steps in care” (provider). Conversely, people with difficulty expressing themselves verbally, perhaps from language barriers, may find SDM more challenging during VC because the “communication mismatch” casts doubt on whether patients and providers are understanding each other, a foundational component of achieving SDM (provider).
Nonverbal communication transmits differently during VC. Virtual platforms emphasize eye contact between patients and providers but limit other nonverbal cues compared with in‐person encounters. This limitation may “accentuate” “cultural, language,” or other verbal communication challenges during VC (patient) such that “it may make sense to be in‐person where you can use visual clues…to get your point across” (provider) and ensure shared understanding.
Sharing information
Patients and providers share information to conduct the steps of SDM, and VC affects relaying clinical data via physical examinations, sharing information among the health care team, and providing patient education.
The virtual physical examination impedes SDM when tactile findings are critical and when patients cannot perform examination maneuvers. Participants recommend in‐person evaluation “if there's been a change in the person's condition… because the physician can't touch” areas under evaluation to conduct necessary physical examination elements virtually (patient). This recommendation includes when RCCs interfere with patients’ ability to provide accurate virtual examination data. A patient with rheumatoid arthritis recalled challenges “picking up [their] camera and trying to…show [their] feet” because their “hands aren't the best,” reducing the quality of clinical information shared with their provider.
Certainty of clinical data influences virtual SDM. When providers are “quite confident in [their] diagnosis,” they easily share their assessment and potential treatment options. “The challenge…[is]…where [providers are] not 100% certain because the symptoms are…borderline” combined with virtual platforms that restrict “touching” and “seeing” findings. These circumstances change SDM into “the hardest thing to do virtually” because uncertainty prevents providers and patients from “being clear on [their] decision.” Patients echo this opinion, agreeing that “as long as you know the status of what's going on, then making decisions…works…as well virtually as it does in person….”
On the other hand, VC can expand information sharing among members of the health care team. Traditional mechanisms, such as forwarding documentation in the electronic health record, notify parties of what occurred. However, VC encounters that include care partners and other providers in multisite visits prospectively integrate stakeholders into SDM, highlighting a potential benefit of VC that may be unattainable in person.
“Many times there's more than one clinician…involved in this decision‐making process….I have had…(virtual) visits with a patient where their nephrologist also joined,…a huge bonus of telehealth.” (provider)
Similarly, virtual education tools, including chat functions, after visit summaries, and screen sharing that presents data and visual aids, enhance SDM. However, participants report not “[having] all the…resources that [they] might want” (provider), identifying opportunities to develop better virtual tools. Ideas include virtual whiteboards and decision‐making aids that convert in‐person practices to VC.
“When I'm in‐person… and sharing decision‐making, I'll…write the name of each…option and then…jot down… pros, cons….Often the patient likes to take that with them…. I don't have a good way to do that over telemedicine….” (provider)
Choosing
The word “choice” underscores patient autonomy when selecting options, a SDM step that can be optimized through VC. Patients require time to choose, and VC maximizes time for making decisions because workflows differ from in‐person encounters.
“In an encounter, it's…15 minutes for a patient to be roomed….You may have…seven minutes talking to a patient….In a telemedicine encounter, you may have 25 minutes to talk to the patient….” (provider)
In VC, decisions can be made over multiple appointments, further expanding time for choosing. “If a patient is not comfortable, following up with…a telemedicine visit” is better than “pushing them into a decision” (provider). Secure messaging offers another platform to continue SDM between appointments. Providers who are “really good about communicating…with [patients] between visits using the portal…help…[in making] major decisions” (patient). For “big decisions,” patients “really value” and feel “supported” when SDM occurs over several encounters using a combination of in‐person and virtual platforms (patient). VC also allows providers to “[circle] back with [patients] at a later date” for updates on patients’ decision‐making or how a treatment is working (provider).
Technology
As an external factor, technology can facilitate or hinder SDM during VC. In addition to sharing information and checking in with patients, technology supports SDM when it minimizes environmental distractions. Patients notice this benefitting providers most.
“The providers listen more when I'm doing a telehealth visit.…In the examination room,…providers look at the computer screen…, but now I am the computer screen!” (patient)
Although technology improves providers’ focus, it exposes patients to environmental distractions, requiring providers to assess “Is the home environment suitably private? [Are there] enough distractions that the patient can't concentrate on the encounter?” before proceeding with SDM (provider).
Technology further hinders SDM when people lack access, including “high quality…video and audio connection” (provider). Poor quality telecommunications distract from patient care, impeding communication, and reducing information sharing. Social factors, such as financial security and geographic location, can further limit access to technology, exacerbating disparate health outcomes.
Themes and subthemes regarding external factors generated from qualitative analysis of focus group transcripts with representative quotations from patient and provider participants
- Technology
- Positive influences of technology
- “…some patients don't have access to high quality…video connection….With a high quality video and audio connection, I'm able to have excellent conversations, I'm not distracted, I'm sitting right in front of them, I'm looking straight at them through the camera.” (provider)
- Negative influences of technology
- “It's important to bring up poverty,…geographical location, access to internet and phone…in terms of… social determinants of health….How…that contributes to the current setting of a pandemic, where people who are in those settings have to go into the office more and are more likely to be exposed and also more likely to have poor outcomes from COVID.” (patient)
- Health care system
- Patient access to care
- “I had some symptoms…regarding gout.…It was just…an email…that connected…my rheumatologist to me…to schedule appointments….I see telehealth as an avenue to…maximize appointments….I would've missed a lot of appointments…because I can't always make myself readily available in person….The online meetings actually come to the rescue, and I can…keep…my appointment and…follow through my regimen, all thanks to telehealth.” (patient)
- Health policies
- “…when it comes to orthopedic care… that's very hard to do unless you have imaging.…You're already having to leave the house for imaging…which is a disadvantage…. My insurance…they're not gonna pay for virtual care anymore…. I'm very much in a panic of, ‘Oh, I can't see the doctors that I wanna see that are covered by my insurance.’” (patient)
- Workflow
Unfamiliarity with requisite VC technology reduces SDM, and learning to use it can be stressful. Participants note that technologic support and contingencies embedded into VC workflows reduce user anxiety while optimizing SDM.
“Offering…extra support…helps…reduce anxiety….It can…be like,… ‘Well, I don't know if this location has enough internet access for me to get the critical information I need to give my doctor right now so that he can give me critical meds that I need today.’…One of the things that…would be really helpful is if they would…be like, ‘Let me…make sure this is your correct phone number. If we lose…connection, I can call you….’” (patient)
An inability to use technology impairs telecommunications, and those who “are not necessarily tech savvy” may experience impaired SDM during VC (patient). For example, a patient “tried to plug [their] headphones in, and it wasn't working,” exclaiming “‘You guys are having a conversation, and…I can't hear anything!’” Instances like these prevent effective communication, further reducing the trust, information sharing, and choice essential for virtual SDM.
Health care system
The health care system, defined as the medical system, insurance structures, and health policies, represents another external factor that influences SDM during VC. VC supports SDM when it enhances health care access despite distance, illness, and social and structural barriers. A patient emphasized this relationship, commenting the VC maintains their access to care. On the other hand, participants cited several instances when the health care system prevented access to virtual SDM, including concerns of insurance coverage, as well as treatment and medical licensing restrictions.
“Medicare requires one in‐person visit per year for each provider that prescribes…controlled substances….As a chronic pain patient who takes an opioid, as someone who takes an anxiety med that is controlled,…as a transgender person who takes a controlled hormonal substance…, that's at least three extra visits in‐person a year … I'm required to do in order to maintain my treatment.” (patient)
“It was…my…primary care telemedicine appointment….We ended up going to Chicago, [Illinois]….They were asking, ‘Are you in…Wisconsin?’… I'm like, ‘No, I'm only two hours away….’ I had to reschedule.” (patient)
The systems within individual clinics influence patients’ participation in SDM during VC by optimizing access to care and increasing information available for SDM. Patients and providers benefit when virtual workflows streamline scheduling. For example, “an email… connected… a rheumatologist to [a patient]… to schedule appointments” (patient), whereas “the capability for clinicians…to…schedule [their] own telemedicine visits” reduces administrative burden (provider). Workflows that procure diagnostic studies before virtual appointments increase the yield of virtual SDM, as results can be integrated into the conversation. “It is challenging…if people have to come in multiple times…” for diagnostic studies (provider). Practices with “a strong preference to get labs [and other diagnostic data] first” proactively overcome this barrier and reinforce information sharing during virtual SDM (provider).
DISCUSSION
Our study is the first to investigate the benefits, shortcomings, and optimization of SDM for RCCs during VC. Patients and providers identify SDM as an essential component of VC, and the same general process, grounded in patient‐provider relationships, occurs whether SDM is conducted virtually or in person. VC overcomes known barriers to SDM, expanding time for decision‐making, improving health literacy through patient education, and enhancing access to care. VC introduces new barriers to SDM because of increased reliance on verbal communication, challenges with the virtual physical examination, and unfamiliarity with or restricted access to technology. Therefore, VC provides opportunities for SDM that are sometimes more and sometimes less optimal than in‐person options; patients and providers must develop skills to differentiate between these scenarios and most suitably use VC in SDM.
SDM is a complex process using multiple skills related to communication, interpersonal relationships, clinical reasoning and health literacy, and health systems. 17 , 18 These skills concomitantly inform the overall clinical context, creating an inextricable link between factors influencing the encounter and factors affecting SDM. Our results affirm this connection, and many provided examples regarding SDM during VC align with practices affecting the general use of VC. 9
Trusting patient‐provider relationships are best established in person, and the time needed to develop trust is unique for each patient and provider. This phenomenon recurs in medicine, as patients reported difficulty establishing rapport with their primary care providers during VC. 19 Although the specific reason remains unknown, diminished nonverbal communication and the virtual physical examination likely contribute. Nonverbal cues carry messages 20 and building relationships can be difficult without them. The ancillary clinical environment and friendliness of clinic staff contribute to nonverbal communication but are removed from VC. Furthermore, relationships and RCCs may move through stages of trust and skepticism, as well as symptom relapse and control, with periods of skepticism or relapse requiring more intensive care and an in‐person approach to SDM. Additional challenges emerge during virtual physical examinations, preventing patients from sharing clinical information. In response, researchers are developing tools that guide patients to conduct physical examination elements. 21 Meanwhile, patients and providers should continue establishing relationships and baseline physical examination findings in person before transitioning to VC and tending to situations when in‐person SDM may prove more efficacious.
Patients who identify with underrepresented groups experience additional challenges establishing trust with their providers, similar to studies exploring in‐person SDM. 22 Virtual SDM can be successful when respect, listening, and cultural humility are maintained and trusting patient‐provider relationships are formed first. Given the importance of establishing these relationships in person, additional in‐person encounters may be completed to solidify trust before transitioning SDM to VC.
The importance of establishing patient‐provider relationships with underrepresented patients, coupled with considerations regarding access to technology, emerged as factors uniquely affecting SDM during VC for marginalized people. This suggests that most needs for SDM during VC are shared among patients and that processes implemented to address other barriers will benefit underrepresented groups too.
Time is a known barrier to SDM, 8 and VC overcomes the challenge. VC maximizes patients’ time for decision‐making by minimizing time spent on registration and rooming, providing platforms that extend SDM across multiple encounters, and strengthening follow‐up after making decisions. When VC expands the time available for patients to consider their options, it enhances the delivery of patient‐centered care.
Patients benefit when education tools are embedded within VC, but some tools have not yet transferred to VC. Experts could design and validate mechanisms for drawing or outlining concepts virtually, as well as virtual decision‐making aids. 23 Another opportunity may include creating sound bites depicting complex concepts like disease processes or treatment mechanisms that could be played during VC encounters. Such resources would benefit the health literacy of many patients and should be available for public use.
As VC integrates technology into care delivery, providers must demonstrate structural humility, recognizing situations that would disadvantage SDM because of patient unfamiliarity with or access to technology. Health care systems also must incorporate resources, such as patient instructions for VC platforms and technology support embedded within virtual appointments, to reduce barriers to virtual SDM. Additional resources may prepare patients for managing the aspects of VC that differ from in‐person encounters. For example, CreakyJoints, an organization that supports people with arthritis, created eRheum (https://erheum.org/) to help patients prepare for VC visits. Providers are prudent to integrate these resources into standard workflows, orienting patients to VC and optimizing SDM.
Even though the steps of SDM are the same in person and virtually, education that enhances provider capacity to conduct them during VC will benefit SDM. Such initiatives would reinforce established telehealth competencies while describing nuances of how virtual skills apply to SDM. 24 , 25 Instructional activities might highlight options for optimizing verbal communication when announcing decisions, listing options, and eliciting patient preferences. Materials could also introduce choosing when to conduct SDM virtually versus in person, leveraging virtual patient education tools, or developing systems that support access to virtual SDM.
The design of our study strengthens the trustworthiness and transferability of our results. We created a research team with diverse experiences in primary care, rheumatology, patient advocacy, medical anthropology, education, and qualitative methods that enriched the analysis of interview transcripts. Similarly, study participants represented diversity in underlying diagnoses, geographic location, and underrepresented groups. Lastly, we asked participants to review a summary of our findings and provide their input to reinforce and refine the trustworthiness of our analysis.
Limitations of our study include participant backgrounds. Most participants had inflammatory arthritis, worked within the field of rheumatology, and lived in the Midwest, a reflection of our partnership with AiArthritis, an inflammatory arthritis patient advocacy group with headquarters in Missouri. Furthermore, our population underrepresents Asian and Pacific Islander participants. It is possible that new themes would have emerged if different participants were included, especially if greater international insight informed the theme of health care systems. To overcome this limitation, future research could partner with multiple advocacy groups from different geographic regions and specialties. Second, the presence of researchers during focus groups may have influenced participants’ responses even though a physician and patient from the research team conducted interviews to help patients feel comfortable sharing their insights. Third, the collaboration in focus groups may have limited nuanced results, and researchers might have elicited information more specific to SDM during VC in one‐on‐one interviews. Lastly, people enthusiastic about VC likely volunteered to participate, which may overemphasize findings favoring virtual SDM.
SDM is an essential component of health care, supporting patient autonomy, medical adherence, and health equity. VC, a mainstay of care delivery, provides a virtual platform for SDM while also offering solutions for barriers to SDM. In order to maximize the benefits of SDM, multiple facets of health care systems must optimize VC workflows and education tools while maintaining patient access to VC. In doing so, patients, providers, insurance carriers, and policy makers will enhance the care of RCCs.
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Dr. Zickuhr had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design
Zickuhr, McCarthy, Nolan‐Thomas, Westrich‐Robertson, Hanson.
Acquisition of data
Zickuhr, McCarthy, Nolan‐Thomas, Westrich‐Robertson.
Analysis and interpretation of data
Zickuhr, McCarthy, Nolan‐Thomas, Westrich‐Robertson, Baker, Hanson.
Supporting information
Disclosure form
Supplementary 1 Focus Group Semi‐Structured Interview Guide
Supplementary 2
ACKNOWLEDGMENTS
We thank the patients and providers who participated in focus groups for their willingness to share their experiences and suggestions for optimizing shared decision‐making during virtual care.
Funded by the Rheumatology Research Foundation Clinician Scholar Educator award (grant CSE212223).
Additional supplementary information cited in this article can be found online in the Supporting Information section (http://onlinelibrary.wiley.com/doi/10.1002/acr2.11633).
Author disclosures are available at https://onlinelibrary.wiley.com/doi/10.1002/acr2.11633.
REFERENCES
- 1. Stiggelbout AM, Pieterse AH, De Haes JC. Shared decision making: concepts, evidence, and practice. Patient Educ Couns 2015;98:1172–1179. [DOI] [PubMed] [Google Scholar]
- 2. Durand MA, Carpenter L, Dolan H, et al. Do interventions designed to support shared decision‐making reduce health inequalities? A systematic review and meta‐analysis. PLoS One 2014;9:e94670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fiorillo A, Barlati S, Bellomo A, et al. The role of shared decision‐making in improving adherence to pharmacological treatments in patients with schizophrenia: a clinical review. Ann Gen Psychiatry 2020;19:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hoffmann TC, Légaré F, Simmons MB, et al. Shared decision making: what do clinicians need to know and why should they bother? Med J Aust 2014;201:35–39. [DOI] [PubMed] [Google Scholar]
- 5. Hughes TM, Merath K, Chen Q, et al. Association of shared decision‐making on patient‐reported health outcomes and healthcare utilization. Am J Surg 2018;216:7–12. [DOI] [PubMed] [Google Scholar]
- 6. Légaré F, Adekpedjou R, Stacey D, et al. Interventions for increasing the use of shared decision making by healthcare professionals. Cochrane Database Syst Rev 2018;7:CD006732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Perez Jolles M, Richmond J, Thomas KC. Minority patient preferences, barriers, and facilitators for shared decision‐making with health care providers in the USA: a systematic review. Patient Educ Couns 2019;102:1251–1262. [DOI] [PubMed] [Google Scholar]
- 8. Hernández‐Leal MJ, Pérez‐Lacasta MJ, Feijoo‐Cid M, et al. Healthcare professionals' behaviour regarding the implementation of shared decision‐making in screening programmes: a systematic review. Patient Educ Couns 2021;104:1933–1944. [DOI] [PubMed] [Google Scholar]
- 9. Li CZ, Borycki EM, Kushniruk AW. Connecting the world of healthcare virtually: a scoping review on virtual care delivery. Healthcare (Basel) 2021;9:1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hawkins AT, Ueland T, Aher C et al. Shared decision‐making in general surgery: prospective comparison of telemedicine vs in‐person visits. J Am Coll Surg 2023;236:762–771. [DOI] [PubMed] [Google Scholar]
- 11. AiArthritis Voices 360 Degrees . The Future of Shared Decision‐Making and E‐Health in Rheumatology Starts with YOU! Accessed April 15, 2023. https://www.aiarthritis.org/talkshow-ep70
- 12. Pappas Y, Vseteckova J, Mastellos N, et al. Diagnosis and decision‐making in telemedicine. J Patient Exp 2019;6:296–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ramsetty A, Adams C. Impact of the digital divide in the age of COVID‐19. J Am Med Inform Assoc. 2020;27:1147–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Harris PA, Taylor R, Minor BL, et al. The REDCap consortium: building an international community of software partners. J Biomed Inform 2019;95:103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hsieh HF, Shannon SE. Three approaches to qualitative content analysis. Qual Health Res 2005;15:1277–1288. [DOI] [PubMed] [Google Scholar]
- 16. Tong A, Sainsbury P, Craig J. Consolidated criteria for reporting qualitative research (COREQ): a 32‐item checklist for interviews and focus groups. Int J Qual Health Care 2007;19:349–357. [DOI] [PubMed] [Google Scholar]
- 17. Kriston L, Hahlweg P, Härter M, Scholl I. A skills network approach to physicians' competence in shared decision making. Health Expect 2020;23:1466–1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Muscat DM, Morony S, Trevena L, et al. Skills for shared decision‐making: evaluation of a health literacy program for consumers with lower literacy levels. Health Lit Res Pract 2019;3:S58–S74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Andreadis K, Muellers K, Ancker JS, et al. Telemedicine impact on the patient‐provider relationship in primary care during the COVID‐19 pandemic. Med Care 2023;61(Suppl 1):S83–S88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mast MS. On the importance of nonverbal communication in the physician‐patient interaction. Patient Educ Couns 2007;67:315–318. [DOI] [PubMed] [Google Scholar]
- 21. Nowell WB, Venuturupalli V. Expert conversations: optimizing care in rheumatology through telehealth. Consultant360. Accessed April 15, 2023. https://www.consultant360.com/exclusive/rheumatology/expert‐conversations‐optimizing‐care‐rheumatology‐through‐telehealth [Google Scholar]
- 22. Peek ME, Gorawara‐Bhat R, Quinn MT, et al. Patient trust in physicians and shared decision‐making among African‐Americans with diabetes. Health Commun 2013;28:616–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lopez‐Olivo MA, Suarez‐Almazor ME. Digital patient education and decision aids. Rheum Dis Clin North Am 2019;45:245–256. [DOI] [PubMed] [Google Scholar]
- 24. Association of American Medical Colleges . New emerging areas in medicine series: telehealth competencies across the learning continuum. Accessed April 15, 2023. https://store.aamc.org/downloadable/download/sample/sample_id/412/
- 25. Zickuhr L, Albert DA, Herndon C, et al. Addressing competency in rheumatology telehealth care delivery. Arthritis Care Res (Hoboken) 2023;75:1213–1219. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Disclosure form
Supplementary 1 Focus Group Semi‐Structured Interview Guide
Supplementary 2


