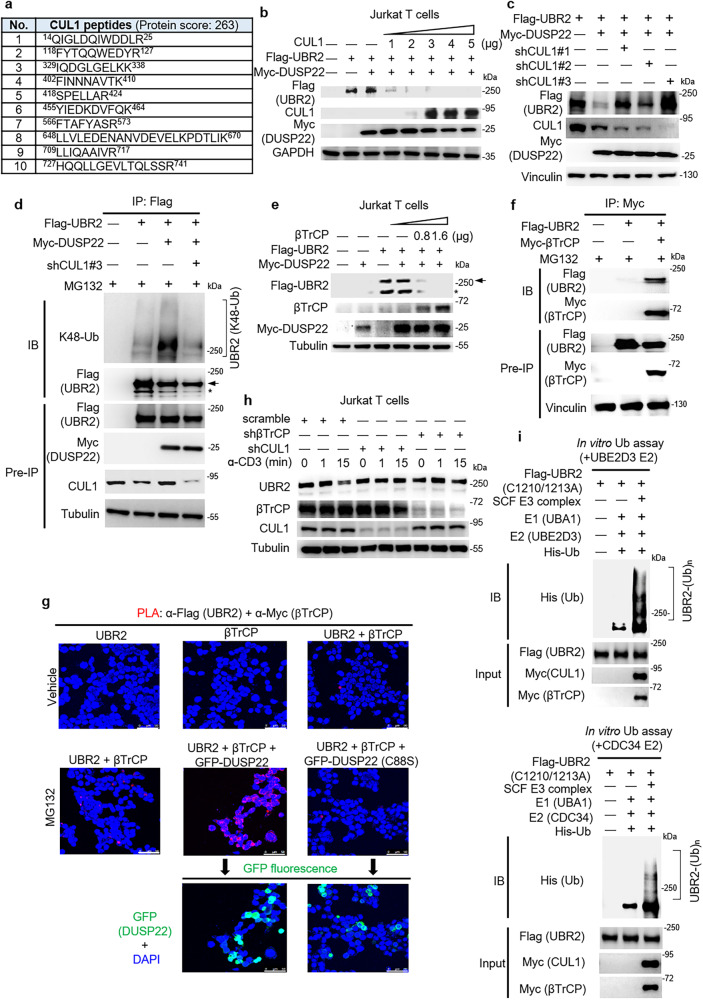
Fig. 3. CUL1-βTrCP E3 ligase complex induces Lys48-linked ubiquitination and degradation of UBR2.
a Identification of CUL1 as an UBR2-interacting protein by mass spectrometry-based proteomics. Identified CUL1 peptides were shown. b Immunoblotting of CUL1, Flag-tagged UBR2, Myc-tagged (DUSP22), and GAPDH proteins in Jurkat T cells co-transfected with Myc-CUL1, Flag-UBR2, and Myc-DUSP22 plasmids. c Immunoblotting of the endogenous CUL1, Flag-tagged UBR2, Myc-tagged DUSP22, and vinculin proteins in HEK293T cells co-transfected with Flag-UBR2, Myc-DUSP22, and CUL1 shRNA plasmids. d CUL1 knockdown inhibited DUSP22-induced UBR2 ubiquitination. Immunoprecipitation and immunoblotting analysis of Lys48-linked ubiquitination of UBR2, Flag-tagged UBR2, Myc-tagged DUSP22, and endogenous CUL1 proteins were performed using the lysates of HEK293T cells co-transfected with Flag-UBR2, Myc-DUSP22, and CUL1 shRNA #3 plasmids. The transfected cells were treated with 25 µM MG132 for 4 h. Arrow, UBR2 protein; asterisk, degraded UBR2 protein. e βTrCP overexpression plus suboptimal DUSP22 (0.8 μg) induced UBR2 degradation. Immunoblotting of Flag-tagged UBR2, Myc-tagged DUSP22, and tubulin proteins in Jurkat T cells co-transfected with Flag-UBR2 and Myc-DUSP22 plus different amounts (0.8 μg, 1.6 μg) of Flag-βTrCP plasmids. f UBR2 interacted with βTrCP. Immunoprecipitation and immunoblotting of Flag-tagged UBR2 with Myc-tagged-βTrCP proteins were performed using the lysates of HEK293T transfected with Flag-UBR2 and Myc-βTrCP plasmids. Anti-vinculin immunoblotting was performed by reprobing the anti-Flag (UBR2) immunoblot membrane. g Confocal microscopy analyses of PLA for the interaction between Flag-tagged UBR2 and Myc-tagged βTrCP proteins in HEK293T cells co-transfected with Flag-UBR2, Myc-βTrCP, and either GFP-DUSP22 or GFP-DUSP22 (C88S) plasmids. Red fluorescence represents the interactions of UBR2 with βTrCP proteins. Images were captured with 400X original magnification. Cell nuclei were stained with DAPI. Scale bar, 50 μm. h Immunoblotting of endogenous UBR2, βTrCP, or CUL1 proteins in Jurkat T cells transfected with scramble shRNAs, βTrCP shRNAs #1, or CUL1 shRNAs #3. T cells were stimulated with anti-CD3 antibody for indicated time periods. i CUL1-βTrCP E3 ligase complex induced UBR2 ubiquitination in vitro. Recombinant His-ubiquitin, E1 (UBA1), E2 (top panel, UBE2D3; bottom panel, CDC34), ATP, and SKP1-CUL1-βTrCP-RBX1 complex were co-incubated in the ubiquitination buffer with Flag-tagged UBR2 E3 ligase-inactive mutant (C1210/1213A) proteins.