Abstract
Background
Trauma pneumonectomy remains an incredibly morbid procedure, reserved for the most critical cases where it is the only surgical option to stop massive ongoing hemorrhage. There are only few cases reported in the literature of survivors of trauma pneumonectomy complicated by acute respiratory distress syndrome (ARDS). We present our case of long-term survival in this circumstance. Given the limited published research on survival after prolonged veno-venous extracorporeal membrane oxygenation (VV-ECMO), it is important to share our experiences using VV-ECMO as an adjunct for pulmonary recovery.
Case Description
We present a case of a 35-year-old male patient who survived a gunshot wound to the right lung following trauma pneumonectomy with the assistance of VV-ECMO. He developed postoperative hemodynamic instability and required 38 days of VV-ECMO. He ultimately survived discharge from the hospital. One year after his gunshot injury, the patient was living at home with assistance. Urgent VV-ECMO cannulation and a multi-disciplinary approach was lifesaving in the treatment of this patient’s post-pneumonectomy ARDS.
Conclusions
In review of the literature, ECMO has been used in a few other cases of ARDS following trauma pneumonectomy to allow for full pulmonary recovery. This case highlights the challenges following this morbid procedure, however with a multidisciplinary approach and urgent use of ECMO, a favorable outcome can be achieved.
Keywords: Pneumonectomy, veno-venous extracorporeal membrane oxygenation (VV ECMO), acute respiratory distress system, multidisciplinary care, case report
Highlight box.
Key findings
• Early institution of extracorporeal membrane oxygenation (ECMO) after trauma pneumonectomy can improve rates of survival.
What is known and what is new?
• ECMO can be used after trauma pneumonectomy for right heart failure and hypotension.
• ECMO can be maintained after trauma pneumonectomy for prolonged periods with a successful outcome. This report is the longest time on ECMO after trauma pneumonectomy with survival to discharge.
What is the implication, and what should change now?
• ECMO should be considered more frequently after trauma pneumonectomy for right heart failure or shock.
Introduction
Trauma pneumonectomy is rarely required for penetrating injuries to the thoracic cavity. Indications for this procedure include treatment of massive hemorrhage when other parenchymal sparing procedures have failed or are unable to be performed, or main bronchus rupture with patient in extremis. When performed mortality approaches 100% due to respiratory insufficiency, shock, and right heart failure (1,2). We present a case report of a patient who underwent trauma pneumonectomy followed by urgent veno-venous extracorporeal membrane oxygenation (VV-ECMO). The patient ultimately did well and was discharged to a rehabilitation facility. We present this case in accordance with the CARE reporting checklist (available at https://acr.amegroups.com/article/view/10.21037/acr-23-76/rc).
Case presentation
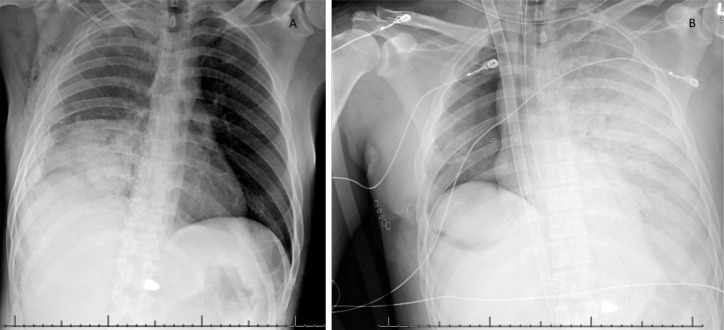
A 35-year-old male presented to the emergency department following a gunshot wound to the right upper chest. He was a previously healthy gentleman with no medical problems and no prior surgeries. Following his gunshot injury, he was transferred from the scene to the emergency room by transport. The initial chest X-ray obtained in the trauma bay is demonstrated in Figure 1A. He was intubated and had a right chest tube placed. Initially 2 liters of frank blood were evacuated, prompting immediate transfer to the operating room by the trauma surgery team. A right thoracotomy was performed, and the bullet trajectory was noted to have traveled from superior to inferior near the pulmonary hilum. Due to ongoing hemorrhage the decision was made by the trauma surgery team to proceed with a pneumonectomy by stapling across the pulmonary hilum in entirety. Individual structures were unable to be dissected and separately ligated due to uncontrolled hemorrhage and patient instability. After the right lung was removed a defect in the bronchial stump was noted, likely was secondary to the mass ligation of the pulmonary hilum. Attempts were made to oversaw the stump with interrupted sutures, however given continued hemodynamic instability, the chest was kept open, and he was transferred to the intensive care unit for further resuscitation.
Figure 1.
Chest X-ray on arrival to trauma bay immediately following gunshot wound to right chest (A) and post-operative day 2 demonstrating acute respiratory distress syndrome in the remaining left lung (B).
Post-operatively the thoracic surgery team was consulted for management of the injured bronchial stump. However, on post-operative day (POD) 2, the patient experienced sudden worsening hypoxia despite maximum ventilatory support. Chest X-ray appearance (Figure 1B) was consistent with acute respiratory distress syndrome (ARDS) prompting initiation of emergent VV-ECMO via jugular dual lumen catheterization.
In the ensuing days, the patient had progressive aeration of the left lung as seen on serial radiographs. His right chest was packed with gauze and changed daily. The packing reduced the air leak to a minimal level and helped to stabilize his respiratory mechanics. He was initially managed with a single lumen endotracheal tube that was advanced into the left mainstem bronchus. On POD 13, a bedside bronchoscopy revealed partial dehiscence of the bronchial stump. As he was anticoagulated for VV-ECMO with ongoing hemodynamic instability, a modified Y-stent was placed during bronchoscopy by interventional pulmonology within the trachea. The stent was fashioned and placed by the pulmonology team to cover the entire right mainstem bronchial stump. The right lumen of the Y-stent was sutured closed with a bioprosthetic patch to seal off the right hemithorax. The endotracheal tube was withdrawn to the distal trachea, above the stent, to avoid manipulation and migration of the stent.
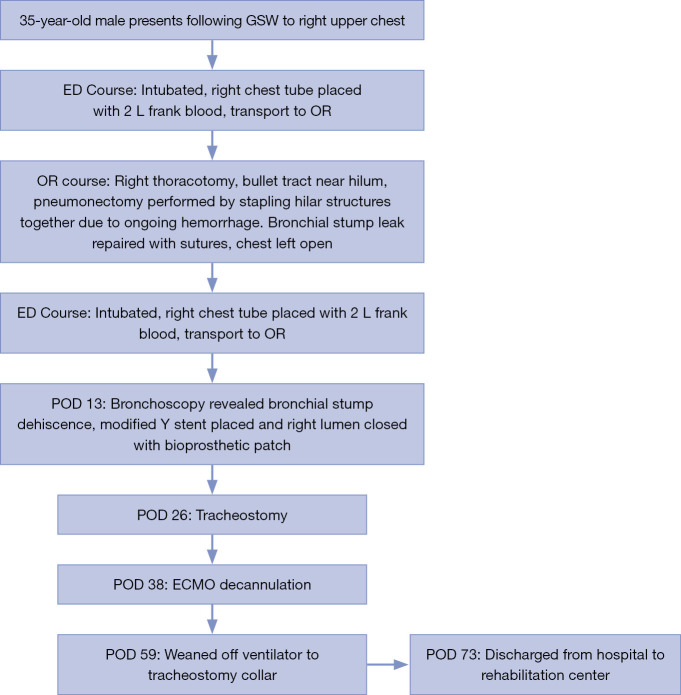
The patient continued to improve from both a hemodynamic and respiratory standpoint, and a tracheostomy was able to be performed on POD 26 with ECMO decannulation on POD 38. His oxygen requirements further decreased, and he was weaned to a tracheal collar by POD 59. He continued to recover with eventual discharge on POD 73 to a rehabilitation center. His clinical course is described in Figure 2.
Figure 2.
Patient clinical course timeline. GSW, gunshot wound; ED, emergency department; OR, operating room; POD, post-operative day; ECMO, extracorporeal membrane oxygenation.
Six months after his gunshot injury, the patient had a myocutaneous flap placed to cover the defect from the thoracotomy wound. By this time the bronchopleural fistula had resolved and the stent was able to be removed. His tracheal device had been removed and he did not require supplemental oxygen. On follow up 1 year after the injury, the patient was living at home with continued care from home nursing and physical therapy.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for the publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
Trauma pneumonectomy is a highly morbid procedure but can be performed successfully with multidisciplinary support. In our case, our patient had input from various teams of providers allowing him to be discharged successfully following his injury. Pneumonectomy in the trauma setting should be performed only when necessary. If required, however, it is preferable to divide the hilar structures individually. Given the hemodynamic instability and ongoing hemorrhage, parenchymal sparing techniques, (i.e., tractotomy) were not attempted as the most important goal was to prevent exsanguination.
When pneumonectomy is performed emergently for penetrating trauma, the most common mechanisms of death are post-operative pulmonary edema and ARDS (3). In addition, acute right ventricular failure can lead to hemodynamic instability. It is likely that a combination of acute right ventricular failure and ARDS caused the profound hypotension in our patient. VV-ECMO support in these patients may ultimately be required and early cannulation should be considered to prevent these sequelae (4,5). In review of the literature, few reports of this treatment exist, however among case reports the recurrent recommendation remained to consider advanced support with ECMO early in the patients’ course for best survival outcome (6,7). Without expeditious VV-ECMO cannulation, our patient would have had a low likelihood for survival. Given the high mortality and incidence of post-operative respiratory insufficiency following trauma pneumonectomy, VV-ECMO should be considered as an early adjunct.
In review of the literature, there are few documented successful outcomes of trauma pneumonectomy followed by VV-ECMO. To our knowledge, our case is the longest interval on ECMO in a patient who survived to discharge at 37 days. In our literature review, using key words “ECMO” and “trauma pneumonectomy”, 19 articles were identified, of which 6 addressed ECMO used as an adjunct following trauma pneumonectomy. One additional study commented on ECMO use following a traumatic bronchial injury and was included in our review. The summary of available literature is demonstrated in Table 1 (1-7). Due to the lack of robust literature available, long-term outcomes are difficult to quantify as we likely are not capturing the patients who do not survive trauma pneumonectomy followed by ECMO support. Thus, further work is needed to understand the full realm of patient outcomes following this injury.
Table 1. Relevant literature of ECMO use in patients undergoing trauma pneumonectomy.
| Title | Authors | Year | Review |
|---|---|---|---|
| Extracorporeal membrane oxygenation for severe hypoxemia after trauma pneumonectomy | Rosenthal et al. (2) | 2009 | Single case report of 42-year-old male following gunshot wound to right anterior chest who underwent trauma pneumonectomy and decompressive laparotomy with massive transfusion. Initiated on veno-arterial ECMO day of injury secondary to persistent hypoxemia confirmed on ABG. Patient able to be weaned from ECMO 48 hours post cannulation. Patient eventually discharged home following lengthy hospital stay with costs exceeding $1 million |
| Traumatic pneumonectomy: a viable option for patients in extremis | Halonen-Watras et al. (1) | 2011 | Retrospective trauma registry review at single level 1 trauma center institution. Identified 7 patients over a 6.5 years period who underwent trauma pneumonectomy. Two patients died perioperatively, leaving five short term survivors. Three short term survivors failed conventional ventilatory support and oscillator support and were placed on ECMO circuit. Two patients who received ECMO survived. The third patient who received ECMO developed mesenteric ischemia and died on post-operative day 18 |
| Veno-venous ECMO in ARDS after post-traumatic pneumonectomy | Martucci et al. (5) | 2013 | Single case report of 25-year-old male following major blunt trauma with right main bronchial disruption requiring right pneumonectomy. Patient developed ARDS and placed on veno-venous ECMO support. Course complicated by septic shock on POD 12 necessitating changing of the ECMO circuit; additionally patient with slow lung recovery and pronated on POD 20. Patient weaned from ECMO circuit on POD 29, and subsequently discharged with tracheostomy able to walk and feed himself |
| Nonoperative Damage Control: The Use of Extracorporeal Membrane Oxygenation in Traumatic Bronchial Avulsion as a Bridge to Definitive Operation | Schmoekel et al. (7) | 2016 | Single case report of 35-year-old female following ejection from motor vehicle following a motor vehicle accident. Injuries included a traumatic left lower lobe bronchial avulsion, and patient was intubated with bilateral tube thoracostomies placed. Despite aggressive resuscitation patient failed to maintain adequate oxygenation, and veno-venous ECMO initiated with lung protective ventilation. Patient able to undergo left thoracotomy, left lower lobectomy with pedicled muscle flap to left lower lobe bronchial injury for definitive repair on hospital day 4. She was subsequently weaned from ECMO circuit on post-operative day 2. Post-operative course complicated by methicillin-resistant Staphylococcus aureus empyema requiring return to the operating room for pulmonary decortication. She was discharged home with normal exercise tolerance at 6-month follow up |
| Severe thoracic trauma caused left pneumonectomy complicated by right traumatic wet lung, reversed by extracorporeal membrane oxygenation support-a case report | Wang et al. (4) | 2019 | Single case report of 47-year-old male following crushing chest injury from forklift. Injuries included left main bronchial rupture, and patient was intubated with a double lumen endotracheal tube for single lung ventilation to the right. Despite single lung ventilation patient was unable to maintain adequate oxygen saturation, and veno-venous ECMO was initiated. Patient subsequently stabilized on VV-ECMO, allowing for operative intervention with pneumonectomy given the extent of the bronchial rupture. Patient was able to be weaned from ECMO on post-operative day 10, and from mechanical ventilation on post-operative day 20 |
| Pulmonary Complications After Trauma Pneumonectomy | Kazior et al. (3) | 2020 | Single case report of 18-year-old male following right sided gunshot wound to the chest. Injuries included right upper and lower lobe destruction, and right subclavian vein injury. Right trauma pneumonectomy and right subclavian vein ligation performed and chest left open due to patients' ongoing instability. He returned to the operating room later that day for muscle flap over the bronchial stump and chest closure, and was extubated later that day. On post-operative day 3 patient had increased work of breathing and was re-intubated. He subsequently developed post pneumonectomy syndrome and post pneumonectomy pulmonary edema with progressively worsening hypoxia. Patient was initiated on veno-venous ECMO post-operative day 6 due to persistent hypoxia. He returned to the OR on post-operative day 11 where a bronchopleural fistula was identified and treated with revision of the previous muscle flap. He was subsequently decannulated from ECMO on post-operative day 18. He was discharged to a skilled nursing home on post-operative day 44 and at 1 year follow up has not experienced any additional major complications |
| Penetrating thoracic injury requiring emergency pneumonectomy supported with two ECMO runs: A testament to multidisciplinary critical care medicine | Dotiwala et al. (6) | 2023 | Single case report of 24-year-old male following two gunshot wounds to the chest. Following bilateral chest tube placement, he was taken to the operating room for emergent thoracotomy where a proximal right hilar injury was noted with inability to control the hemorrhage. A right trauma pneumonectomy was performed, following which an additional injury to the mediastinum at the location of the confluence of the azygous vein and SVC was noted and repaired with a single suture. Following operation, he experienced persistent hypoxia and veno-venous ECMO was initiated. Patient experienced improvement in oxygenation and was extubated to nasal cannula, and subsequently de-cannulated post-operative day 4. Hours following decannulation he had an aspiration event secondary to emesis requiring immediate intubation. He continued to decompensate and was placed back on VV-ECMO. He was decannulated from his second VV-ECMO run 23 days post cannulation, and discharged from the intensive care unit on post-operative day 44 to inpatient rehab. At 3-month follow up patient was noted to be working full time with minimal limitations |
ECMO, extracorporeal membrane oxygenation; ABG, arterial blood gas; ARDS, acute respiratory distress syndrome; POD, post-operative day; OR, operating room; VV, veno-venous.
The ability to perform ECMO following traumatic injury needs to be considered in the broader context of medicine delivery. To facilitate an ECMO program, the hospital must be equipped with trained staff members, including surgeons or other clinicians capable of peripheral or central cannulation, staff members including nurses and respiratory therapists familiar with maintaining the ECMO circuit, and additionally the equipment capital needed to run the circuit. Smaller centers who do not have this advanced capability should consider early transfer of patients at high risk for development of post pneumonectomy ARDS to tertiary or quaternary center to give patients the best chance of survival. Centralization of advanced life supporting care, including ECMO, to highly specialized centers may help defray costs from smaller centers as this level of care is expensive. In a study evaluating health care delivery costs for adult patients with coronavirus disease 2019 (COVID-19) undergoing ECMO, median hospital charges exceeded $870,000 and median hospital cost to the patient exceeded $200,000 (8). By equipping the most highly specialized centers with this treatment and establishing a referral pattern from community centers, the health economic impact to the community may be lessened.
This case also highlights the importance of a multidisciplinary approach to caring for these patients. Numerous medical subspecialties were involved including trauma surgery, thoracic surgery, pulmonology, and anesthesia intensivists over the course of his 73-day hospitalization. Without constant involvement and communication amongst all teams, he likely would not have survived. In addition, the bronchopleural fistula was temporized by the pulmonology team with stenting and a bioprosthetic patch. Although stenting is typically not used for this indication, our patient was too unstable to undergo definitive surgical repair in the operating room. This further example of multidisciplinary management led to our patient ultimately surviving his injury.
This report is limited by the inherent nature of a single case report. We feel, however, that reporting this case will assist other clinicians in applying these principles to optimize survival from trauma pneumonectomy. This report is also limited by the development of a bronchopleural fistula secondary to mass stapling of the hilum without dissecting the individual hilar structures. The mass stapling was required to prevent exsanguination, but the fistula likely exacerbated his overall clinic condition. But there is only sparse literature regarding patient survival following trauma pneumonectomy with ECMO support. As such, we propose that this treatment should be considered in similar cases as a method to address severe post-pneumonectomy ARDS. Implementing this treatment will require clinicians and centers to consider early transfer of patients exhibiting hypoxemia following pneumonectomy, and collaboration amongst providers to determine patient eligibility for ECMO cannulation and delivery of advanced life supporting treatment. With such treatment, patient outcomes may be enhanced following severe traumatic pulmonary injury.
Conclusions
We present a unique case of penetrating chest trauma necessitating trauma pneumonectomy for control of life-threatening hemorrhage. Following pneumonectomy, patient was quickly placed on VV ECMO for pulmonary support to allow for remaining lung recovery in the setting of ARDS. We demonstrate in this case ECMO cannulation following trauma pneumonectomy can provide time for pulmonary recovery and survival. By anticipating the pulmonary complications and employing a multidisciplinary approach, the mortality from trauma pneumonectomy can be lessened.
Supplementary
The article’s supplementary files as
Acknowledgments
This case will be presented at CHEST 2023 Annual Meeting, Honolulu, HI.
Funding: None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for the publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Footnotes
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://acr.amegroups.com/article/view/10.21037/acr-23-76/rc
Peer Review File: Available at https://acr.amegroups.com/article/view/10.21037/acr-23-76/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://acr.amegroups.com/article/view/10.21037/acr-23-76/coif). The authors have no conflicts of interest to declare.
References
- 1.Halonen-Watras J, O'Connor J, Scalea T. Traumatic pneumonectomy: a viable option for patients in extremis. Am Surg 2011;77:493-7. 10.1177/000313481107700430 [DOI] [PubMed] [Google Scholar]
- 2.Rosenthal A, McKenney M, Sanchez R, et al. Extracorporeal membrane oxygenation for severe hypoxemia after trauma pneumonectomy. Am Surg 2009;75:1258-60. 10.1177/000313480907501224 [DOI] [PubMed] [Google Scholar]
- 3.Kazior MR, Streams JR, Dennis BM, et al. Pulmonary Complications After Trauma Pneumonectomy. J Cardiothorac Vasc Anesth 2020;34:1952-61. 10.1053/j.jvca.2020.01.057 [DOI] [PubMed] [Google Scholar]
- 4.Wang FY, Fang B, Yu ZH, et al. Severe thoracic trauma caused left pneumonectomy complicated by right traumatic wet lung, reversed by extracorporeal membrane oxygenation support-a case report. BMC Pulm Med 2019;19:30. 10.1186/s12890-019-0790-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martucci G, Panarello G, Bertani A, et al. Veno-venous ECMO in ARDS after post-traumatic pneumonectomy. Intensive Care Med 2013;39:2235-6. 10.1007/s00134-013-3116-4 [DOI] [PubMed] [Google Scholar]
- 6.Dotiwala A, Kalakoti P, Grier LR, et al. Penetrating thoracic injury requiring emergency pneumonectomy supported with two ECMO runs: A testament to multidisciplinary critical care medicine. Trauma Case Rep 2023;44:100779. 10.1016/j.tcr.2023.100779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schmoekel NH, O'Connor JV, Scalea TM. Nonoperative Damage Control: The Use of Extracorporeal Membrane Oxygenation in Traumatic Bronchial Avulsion as a Bridge to Definitive Operation. Ann Thorac Surg 2016;101:2384-6. 10.1016/j.athoracsur.2015.08.033 [DOI] [PubMed] [Google Scholar]
- 8.Mazzeffi M, Curley J, Gallo P, et al. Variation in Hospitalization Costs, Charges, and Lengths of Hospital Stay for Coronavirus Disease 2019 Patients Treated With Venovenous Extracorporeal Membrane Oxygenation in the United States: A Cohort Study. J Cardiothorac Vasc Anesth 2023;37:1449-55. 10.1053/j.jvca.2023.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as