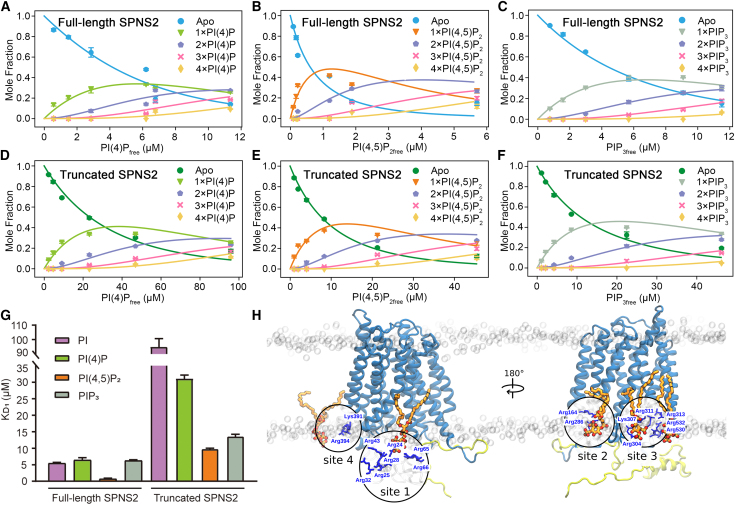
Figure 2.
Determination of binding affinities for PI derivatives to full-length and truncated SPNS2 and potential PI(4,5)P2 binding sites on full-length SPNS2
(A–F) Binding curves for PI(4)P, PI(4,5)P2, and PIP3 binding to full-length SPNS2 (A, B, and C, respectively) and to truncated SPNS2 (D, E, and F, respectively). Data are plotted as mean ± SD (n = 3).
(G) KD1 values for PI, PI(4)P, PI(4,5)P2, and PIP3 binding to full-length and truncated SPNS2. Data are plotted as mean ± SD (n = 3).
(H) MD simulation reveals PI(4,5)P2 molecule (orange sticks) bound to the N terminus (binding site 1), and further three PI(4,5)P2 binding sites are shown (sites 2–4). SPNS2 is shown in blue (with the N terminus colored in yellow), the potential PI(4,5)P2 interacting residues are shown as blue sticks, and phosphorus atoms in the headgroups of membrane lipids are shown as gray spheres.