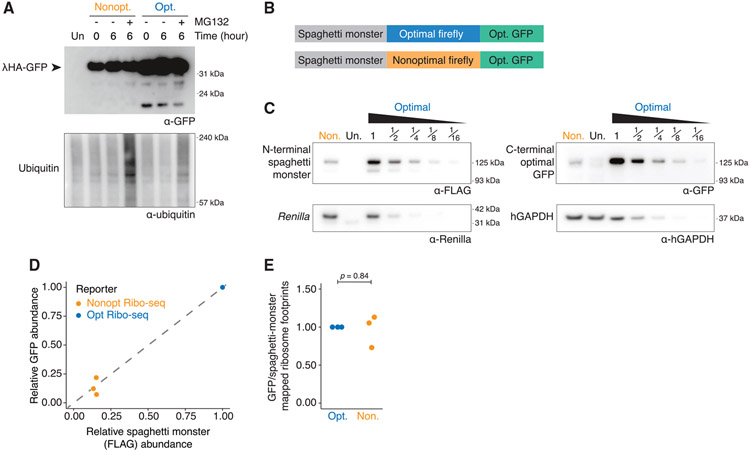
Figure 4. Nonoptimal codons do not lead to incomplete translation or RQC.
(A) Nonoptimal codons do not lead to truncated protein products. HEK239T cells were transfected with lHA-GFP optimality reporters and treated for 6 h with MG132 (10 μM) or DMSO before western blotting and probing for each reporter (α-GFP) and ubiquitin. Four times more nonoptimal γHA-GFP lysate was loaded, and the blot was overexposed.
(B) Schematic of the dual-tagged reporters used for the Ribo-seq experiment.
(C) Nonoptimal codons repress protein expression for the dual-tagged firefly luciferase reporters. HEK293T cells were transfected with the dual-tagged reporters alongside Renilla luciferase, and western blotting determined protein abundance (N-terminal SM tag [α-FLAG]), the C-terminal tag (α-GFP), Renilla luciferase, and hGAPDH. Lysates containing the optimized Ribo-seq reporter were serially diluted, and twice as much nonoptimal Ribo-seq reporter lysate was loaded.
(D) The decrease in FLAG and GFP signals from the nonoptimal dual-tagged Ribo-seq optimality reporter, compared to the optimal, is consistent. Scatterplot is a quantification of n = 3, one of which is shown in (C); p = 0.86.
(E) Nonoptimal codons do not lead to ribosome drop-off. Shown is the ratio of ribosome-protected fragments mapping to the C-terminal GFP tag relative to the N-terminal SM tag for both. The first 25 codons of the SM tag and the last 25 codons of the GFP tag were excluded from this analysis (n = 3).