Abstract
Background:
Human infections with the avian influenza A(H7N9) virus were first reported in China in 2013 and continued to occur in annual waves. In the 2016/2017 fifth wave, Yangtze River Delta (YRD) lineage viruses, which differed antigenically from those of earlier waves, predominated.
Methods:
In this phase 2 double-blinded trial we randomized 720 adults ≥19 years of age to receive two injections of a YRD lineage inactivated A/Hong Kong/125/2017 fifth-wave H7N9 vaccine, given 21 days apart, at doses of 3.75, 7.5, and 15 μg of hemagglutinin (HA) with AS03A adjuvant and at doses of 15 and 45 μg of HA without adjuvant.
Results:
Two doses of adjuvanted vaccine were required to induce HA inhibition (HI) antibody titers ≥40 in most participants. After two doses of the 15 μg H7N9 formulation, given with or without AS03 adjuvant, the proportion achieving a HI titer ≥40 against the vaccine strain at 21 days after the second vaccination was 65% (95% CI, 57%−73%) and 0% (95% CI, 0%−4%), respectively. Among those who received two doses of the 15 μg adjuvanted formulation the proportion with HI titer ≥40 at 21 days after the second vaccination was 76% (95% CI, 66%−84%) in those 19–64 years of age and 49% (95% CI, 37%−62%) in those ≥65 years of age. Responses to the adjuvanted vaccine formulations did not vary by HA content. Antibody responses declined over time and responses against drifted H7N9 strains were diminished. Overall, the vaccines were well tolerated but, as expected, adjuvanted vaccines were associated with more frequent solicited systemic and local adverse events.
Conclusions:
AS03 adjuvant improved the immune responses to an inactivated fifth-wave H7N9 influenza vaccine, particularly in younger adults, but invoked lower responses to drifted H7N9 strains. These findings may inform future influenza pandemic preparedness strategies.
Clinical trials registration:
Keywords: avian influenza virus, vaccine, adjuvants
INTRODUCTION
In March 2013, the first human infections with a novel avian influenza A (H7N9) virus were reported in mainland China.1, 2 Following this initial emergence, annual epidemic waves of human infection occurred until 2017, with case counts peaking in the winter months.3, 4 Most infections were associated with recent poultry exposure4, and no evidence of sustained person-to-person spread of H7N9 was found, although limited person-to-person spread occurred.2, 5–10 Infection was associated with a 40% case-fatality rate,4 and the potential for viral adaptation that would facilitate person-to-person transmission was a major concern.11, 12
The fifth, and as of 2023 the last, wave of human infections with influenza A (H7N9) in mainland China, from October 1, 2016 through September 30, 2017, was the most severe,3, 13 with the number of confirmed cases comparable to that in the previous four waves combined.3, 13 In the fifth wave, a new influenza A(H7N9) lineage predominated. This lineage, designated as Yangtze River Delta (YRD),14 is antigenically distinct from the initial Pearl River Delta (PRD) lineage3 and accounted for over 90% of circulating fifth-wave H7N9 viruses. In addition, unlike previous waves, highly-pathogenic avian influenza (HPAI) viruses, that cause increased morbidity and mortality in poultry, were identified, and these viruses accounted for approximately 3% of fifth-wave human infections.3, 15–18
Banked sera specimens from clinical trial participants who received an AS03-adjuvanted vaccine directed against the 2013 first wave influenza A(H7N9) candidate vaccine virus (CVV)19 were evaluated for cross-reactive responses to the fifth-wave strains. This evaluation showed reduced cross-reactive hemagglutination inhibition (HI) and microneutralization (MN) antibody titers to fifth-wave YRD lineage and HPAI viruses, compared with titers to the 2013 CVV, suggesting the 2013 vaccine induced little cross protection to the fifth-wave viruses.3, 20 Also of concern was the detection fifth wave viral mutations that showed reduced susceptibility to neuraminidase inhibitors.3, 16
Due to the antigenic differences between the fifth-wave YRD viruses and the 2013 CVVs, in March 2017 the World Health Organization (WHO) recommended the development of two new fifth-wave YRD lineage CVVs, including one LPAI strain and one HPAI strain. The WHO Collaborating Center for Reference and Research on Influenza at CDC generated a new H7N9 CVV derived from a YRD lineage LPAI H7N9 virus, A/Hong Kong/125/2017.21 In response to the potential pandemic threat of fifth-wave influenza A(H7N9) viruses, the Vaccine and Treatment Evaluation Unit (VTEU) network, funded by the National Institutes for Allergy and Infectious Diseases (NIAID), rapidly initiated five trials evaluating A/Hong Kong/125/2017 inactivated influenza vaccine (IIV) formulations in healthy adults using antigen and adjuvants procured for the National Pre-pandemic Influenza Vaccine Stockpile by the Biomedical Advanced Research and Development Authority (BARDA), part of the US Department of Health and Human Services (HHS) (clinicaltrials.gov NCT03312231, NCT03682120, NCT03318315, NCT03589807, NCT03738241).22
The first trial, reported here, evaluated a two-dose series of varying amounts of the A/Hong Kong/125/2017 antigen, manufactured by Sanofi, administered with or without AS03A adjuvant, manufactured by GSK, in adults 19 years of age and older, in order to assess the safety and immunogenicity of different dosages of the IIV administered with and without adjuvant in younger and older adults (clinicaltrials.gov NCT03312231).
METHODS
Study design and participants
This randomized, double-blinded, phase 2 study evaluated the immunogenicity and safety of two intramuscular (IM) injections, administered 21 days apart, of an H7N9 IIV given at three dose levels of hemagglutinin (HA) antigen (3.75, 7.5, and 15 μg) combined with AS03A adjuvant (Study Groups 1, 2, and 3) and two dose levels (15 and 45 μg) without adjuvant (Study Groups 4 and 5). After stratification by age group (19–64 and ≥65 years), participants were randomly assigned to one of the five study groups at a ratio of 2:2:2:1:1 (Figure 1).
Figure 1.
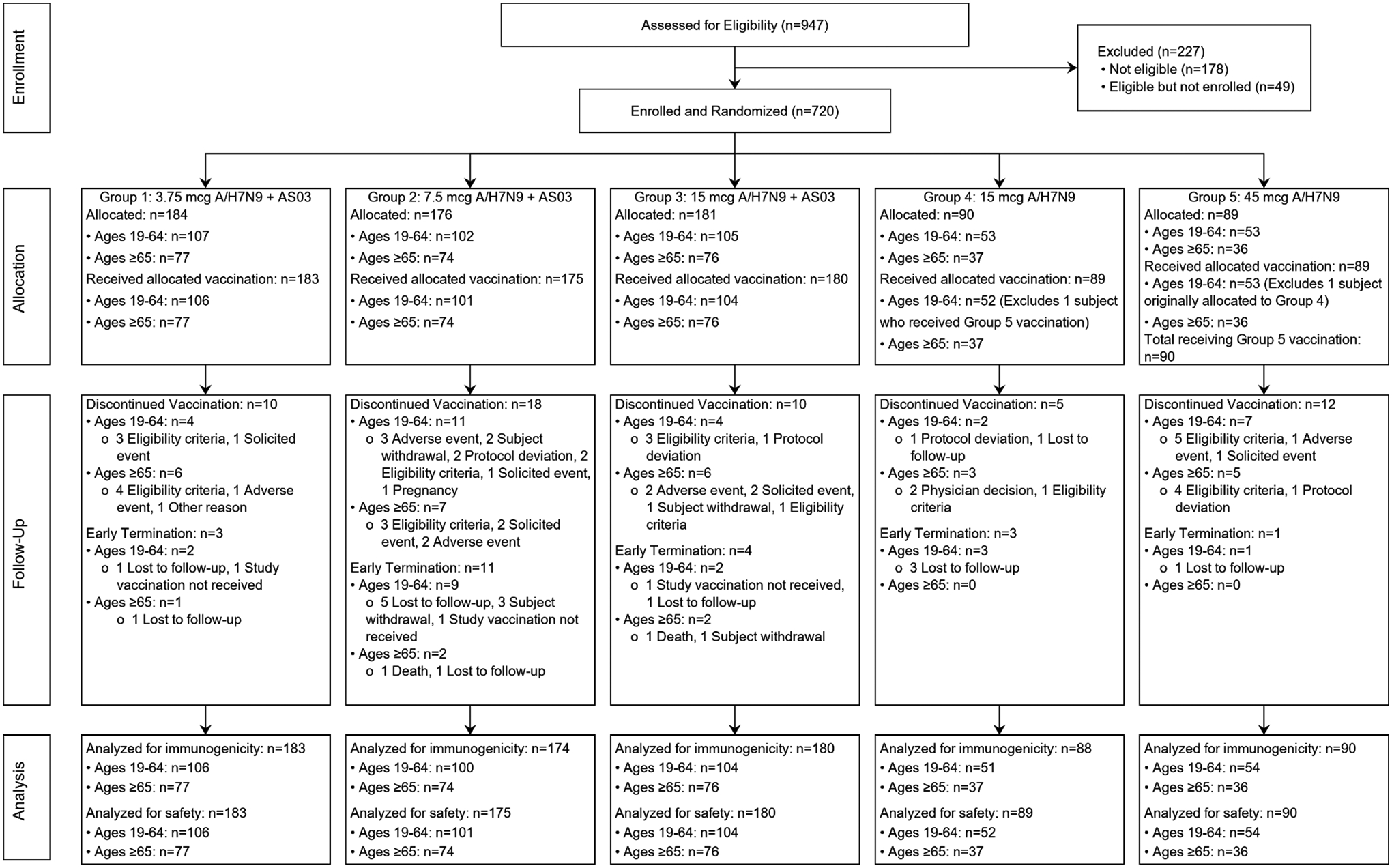
CONSORT Flow Diagram.
One participant was randomized to Group 4 (15 μg without adjuvant) but due to pharmacist error received the Group 5 vaccine (45 μg without adjuvant) for the first vaccination and was then continued with the Group 5 formulation for the second vaccination. In Figure 1 (Consort diagram) and Table 1 (demographics) the participant is included in Group 4, to which they were randomized, but for all safety and immunogenicity analyses is included in Group 5.
Table 1.
Demographic and baseline characteristics of study participants
| Study Group | 1 | 2 | 3 | 4 | 5 | All Participants |
|---|---|---|---|---|---|---|
| Vaccine Formulation | 3.75 μg + AS03 | 7.5 μg + AS03 | 15 μg + AS03 | 15 μg no adjuvant | 45 μg no adjuvant | |
| Number Enrolled | N=184 | N=176 | N=181 | N=89 | N=720 | |
| Gender – n(%) | ||||||
| Male | 89 (48) | 76 (43) | 100 (55) | 42 (47) | 40 (45) | 347 (48) |
| Female | 95 (52) | 100 (57) | 81 (45) | 48 (53) | 49 (55) | 373 (52) |
| Ethnicity – n(%) | ||||||
| Not Hispanic or Latino | 180 (98) | 170 (97) | 171 (94) | 82 (91) | 84 (94) | 687 (95) |
| Hispanic or Latino | 4 (2) | 4 (2) | 9 (5) | 8 (9) | 5 (6) | 30 (4) |
| Not reported | - | 2 (1) | 1 (<1) | - | - | 3 (<1) |
| Race – n(%) | ||||||
| American Indian or Alaska Native | - | - | - | 1 (1) | 1 (1) | 2 (<1) |
| Asian | 7 (4) | 8 (5) | 14 (8) | 1 (1) | 3 (3) | 33 (5) |
| Black or African American | 19 (10) | 22 (13) | 26 (14) | 13 (14) | 10 (11) | 90 (13) |
| White | 150 (82) | 138 (78) | 130 (72) | 70 (78) | 72 (81) | 560 (78) |
| Multiple | 6 (3) | 5 (3) | 7 (4) | 4 (4) | 2 (2) | 24 (3) |
| Unknown | 2 (1) | 3 (2) | 4 (2) | 1 (1) | 1 (1) | 11 (2) |
| Age Categories – n(%) | ||||||
| 19–64 | 107 (58) | 102 (58) | 105 (58) | 53 (59) | 53 (60) | 420 (58) |
| ≥65 | 77 (42) | 74 (42) | 76 (42) | 37 (41) | 36 (40) | 300 (42) |
| Age — year* | ||||||
| 19–64 | 38.6±14.3 | 36.4±12.0 | 36.9±13.0 | 40.3±13.4 | 39.3±13.3 | 37.9±13.2 |
| ≥65 | 71.9±5.4 | 71.4±5.8 | 71.8±5.5 | 71.1±5.1 | 71.1±5.1 | 71.6±5.4 |
| Prior Seasonal Flu Vaccine – n(%) | ||||||
| Neither 2016/2017 nor 2017/2018 | 20 (11) | 28 (16) | 30 (17) | 7 (8) | 10 (11) | 95 (13) |
| 2016/2017 Only | 9 (5) | 10 (6) | 11 (6) | 7 (8) | 4 (4) | 41 (6) |
| 2017/2018 Only | 11 (6) | 9 (5) | 13 (7) | 6 (7) | 5 (6) | 44 (6) |
| Both 2016/2017 and 2017/2018 | 143 (78) | 128 (73) | 125 (69) | 68 (76) | 68 (76) | 532 (74) |
| Unknown | 1 (<1) | 1 (<1) | 2 (1) | 2 (2) | 2 (2) | 8 (1) |
| Body Mass Index (kg/m2) Categories – n(%) | ||||||
| <30 | 134 (73) | 117 (66) | 141 (78) | 67 (74) | 75 (84) | 534 (74) |
| ≥30 | 49 (27) | 59 (34) | 40 (22) | 23 (26) | 14 (16) | 185 (26) |
| Not reported | 1 (<1) | - | - | - | - | 1 (<1) |
Plus–minus values are means ±SD.
Eligible participants were males and non-pregnant females in good health or with controlled chronic illness, without immunosuppression, who were ≥19 years of age and provided written informed consent for study participation. Complete inclusion and exclusion criteria are provided on clinicaltrials.gov (NCT03312231). Participants were enrolled at six VTEU sites between February 14 and September 5, 2018. We evaluated safety and tolerability by identification of serious adverse events (SAEs) and medically-attended adverse events (MAAEs) (including new-onset chronic medical conditions [NOCMCs] and potentially immune-mediated medical conditions [PIMMCs]) from the time of the first study vaccination through approximately 12 months after the last study vaccination; other (nonserious) unsolicited adverse events (AEs) through approximately 21 days after each study vaccination; clinical safety laboratory AEs at 7 days after each vaccination and on the day of (and prior to) the second vaccination; and, using a memory aid, solicited local and systemic AEs through 7 days after each vaccination.
The protocol and informed consent forms were approved by the NIAID Division of Microbiology and Infectious Diseases (DMID), the US Food and Drug Administration, and the institutional review boards of record for each participating study site.
Vaccine and adjuvants
The study vaccine was a monovalent 2017 IIV manufactured from a reverse genetics-derived reassortant CVV IDCDC RG56B (H7N9), containing the HA and neuraminidase genes from the LPAI influenza A/Hong Kong/125/2017 (H7N9) and the PB2, PB1, PA, NP, M, and NS genes from A/Puerto Rico/8/1934 (H1N1). The vaccine was manufactured by Sanofi using a process similar to that used to produce the licensed IIV Fluzone® vaccine. AS03A is an oil-in-water emulsion adjuvant system manufactured by GSK that includes squalene and 11.86 mg α-tocopherol per 0.5 mL dose.
The HA content of the bulk A/H7N9 vaccine formulations was determined by a single radial immunodiffusion assay to be approximately two times higher (14.45, 28.75, and 56.85 μg, respectively, of HA per 0.5 mL dose) than the targeted HA content on the label (7.5, 15, and 30 μg, respectively, of HA per 0.5 mL dose). At each of the study sites, the study vaccine formulations were prepared by research pharmacists, and unblinded staff members who were not involved with subsequent participant follow up administered the vaccine by IM injection in the deltoid muscle.
Preparation of the 7.5 μg and 15 μg adjuvanted formulations involved mixing 0.25 mL of the actual 14.45 μg and 56.85 μg formulations, respectively, with 0.25 mL of adjuvant for administered dosages of 7.225 μg and 14.375 μg of HA per 0.5 mL. Preparation of the 3.75 μg adjuvanted formulation included an initial 1:1 dilution step of the actual 14.45 μg formulation with phosphate buffered saline prior to mixing 0.25 mL of that formulation, containing 7.225 μg of HA, with 0.25 mL of adjuvant for an administered dosage of 3.6125 μg of HA per 0.5 mL.
The 15 μg unadjuvanted vaccine included 0.5 mL of the actual 14.45 μg per 0.5 mL HA formulation. The 45 μg unadjuvanted vaccine was administered as a 0.75 mL volume and was formulated by combining the contents of two of the 0.5 mL vials of the actual 28.75 μg antigen content for an admixture of 57.50 μg per 1.0 mL and then withdrawing 0.75 mL to administer 43.125 μg of antigen. The antigen content of all study vaccine formulations was within 4.2% of the targeted concentration.
Immunogenicity assays
We collected blood samples prior to the first vaccination, at 7 and 21 days after each vaccination, and at 180 days after the second study vaccination. Those samples were tested by qualified HI and MN antibody assays against the homologous A/Hong Kong/125/2017 (H7N9) reassortant virus and the heterologous A/Shanghai/2/2013 and A/Guangdong/17SF003/2016 reassortant viruses at the Southern Research laboratory (Birmingham, Alabama) using methods previously described.23, 24
Statistical analysis
For HI and MN antibodies, the three co-primary immunogenicity outcome measures included the proportion of participants who had an antibody titer of ≥40, the proportion of participants who met the definition of seroconversion (4-fold or greater increase in antibody titer from a baseline titer of ≥10 or a post-vaccination titer ≥40 if the baseline titer was <10), and the geometric mean titers (GMTs) at approximately 21 days after the second vaccination. The secondary immunogenicity outcome measures included the parameters described above at approximately 7, 21, and 28 days after the first study vaccination. Exploratory immunogenicity objectives included assessing responses at 180 days after the second study vaccination, evaluations of the immune responses to two drifted influenza A/H7 viruses (A/Shanghai/2/2013 and A/Guangdong/17SF003/2016), and evaluations of immune responses to the vaccine strain stratified by age, sex, body mass index (BMI), and prior receipt of seasonal influenza vaccines.
For calculation of the GMTs, titers below the limit of detection (titer <10) were assigned a value of 5. Ninety-five percent confidence intervals for the GMT were calculated using the Student’s t-distribution, and exact Clopper-Pearson confidence intervals were calculated for binary endpoints. Statistical significance was considered at a level of α = 0.05 and all tests were two-sided. Analysis was performed using SAS 9.3 (SAS Institute, Cary, North Carolina). As the study was intended to obtain preliminary estimates of immune response and trends between groups, no formal hypothesis testing was planned, thus analyses were not adjusted for multiple comparisons, and no imputation for missing data was performed since missing data were minimal.
As a pre-specified exploratory analysis, logistic regression models were fit to evaluate the association between study group, age (categorical [19–64 and ≥65 years] or continuous by year), sex, BMI (<30 kg/m2 vs. ≥30 kg/m2), and receipt of seasonal influenza vaccine prior to enrollment (no receipt of either the 2016–2017 or the 2017–2018 vaccine vs. prior receipt of either or both vaccines) with the outcome of HI or MN titer ≥40 at 21 or 180 days after the second vaccination. To further evaluate the association of age with antibody responses to vaccine, we examined the correlation of age, as a continuous variable in days, with HI and MN titers against the vaccine strain at 21 days after the second vaccination as an ad hoc analysis.
Logistic regression modeling was also performed to evaluate the relationship between vaccine antigen dose, adjuvant, sex, and age stratum with reporting of solicited local AEs, and with reporting of solicited systemic AEs, following any vaccination in ad hoc analyses. The solicited systemic AE model did not include elevated oral temperature, chills, or nausea, and the solicited local AE model did not include itching, due to lack of sufficient variability in the occurrence of those events to estimate possible differences related to the other variables.
For evaluations of immunogenicity endpoints, the modified intent-to-treat (mITT) analysis subset included data from participants who received at least one dose of study vaccine and contributed both pre- and at least one post- study vaccination venous blood sample for immunogenicity testing. The per protocol analysis subset included all participants in the mITT subset except those who did not receive the second study vaccination, who were found to have been ineligible at baseline, or who had other major protocol deviations. Results of analyses of the two subsets were similar, and only the per protocol analyses are presented. All summaries and analyses of safety data were performed for the Safety Analysis Population, consisting of all participants who received at least one study vaccination and for whom any data on safety were available.
The sample size of at least 160 participants in each of the three adjuvanted study groups, with at least 100 participants in the 19–64 age stratum and 60 participants in the ≥65-year-old age stratum, and at least 80 participants in each of the two unadjuvanted study groups, with at least 50 participants in the 19–64 and 30 participants in the ≥65 year old age stratum, was selected to obtain preliminary estimates of immunogenicity and safety in a time critical manner and was not designed to test any specific null hypothesis.
RESULTS
Enrollment and Demographics
A total of 720 participants were enrolled; of those, 717 participants received the first study vaccination and 662 received the second study vaccination (Figure 1). Demographic and baseline characteristics were similar across study groups (Table 1).
Hemagglutinin and Microneutralization Antibody Responses
At baseline, no participant in the per protocol cohort had an HI antibody titer ≥40, and only one (a participant in the 19–64 age stratum in Group 2) had a MN antibody titer ≥40. Antibody responses were negligible after the first vaccination in all groups (Table 2). Since very few participants had detectable antibody titers against the H7N9 strain at baseline, the primary outcome measures of proportion with an antibody titer ≥40 and the proportion who met the definition of seroconversion were essentially identical. For simplicity, we present only the outcome measure of proportion of participants with a titer ≥40.
Table 2.
Hemagglutination inhibition and microneutralization antibody responses against A/Hong Kong/125/2017 (H7N9) by study day and age stratum
| Group 1 3.75 μg A/H7N9 + AS03A (N=181) | Group 2 7.5 μg A/H7N9 + AS03A (N=168) | Group 3 15 μg A/H7N9 + AS03A (N=178) | Group 4 15 μg A/H7N9 (N=87) | Group 5 45 μg A/H7N9 (N=88) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 19–64 | ≥65 | 19–64 | ≥65 | 19–64 | ≥65 | 19–64 | ≥65 | 19–64 | ≥65 | |
| Day 1 (Pre-Vaccination 1) | Hemagglutination Inhibition Antibody Responses | |||||||||
| n | 106 | 75 | 97 | 71 | 104 | 74 | 51 | 36 | 54 | 34 |
| GMT (95% CI) | 5.1 (5.0, 5.3) | 5.3 (5.0, 5.6) | 5.2 (5.0, 5.3) | 5.1 (5.0, 5.3) | 5.1 (5.0, 5.2) | 5.4 (5.2, 5.6) | 5.2 (5.1, 5.4) | 5.3 (5.0, 5.6) | 5.2 (4.9, 5.4) | 5.6 (5.1, 6.1) |
| Titer ≥40 – % (95% CI) | 0 (0, 3) | 0 (0, 5) | 0 (0, 4) | 0 (0, 5) | 0 (0, 3) | 0 (0, 5) | 0 (0, 7) | 0 (0, 10) | 0 (0, 7) | 0 (0, 10) |
| 7 Days Post Vaccination 1 | ||||||||||
| n | 106 | 75 | 96 | 69 | 104 | 74 | 51 | 36 | 54 | 34 |
| GMT (95% CI) | 5.3 (5.1, 5.5) | 5.4 (5.0, 5.8) | 5.6 (5.1, 6.1) | 5.6 (4.9, 6.5) | 5.6 (5.3, 5.9) | 6.0 (5.3, 6.8) | 5.3 (5.1, 5.6) | 5.3 (5.0, 5.7) | 5.8 (5.2, 6.5) | 5.5 (5.0, 6.0) |
| Titer ≥40 – % (95% CI) | 0 (0, 3) | 1 (0, 7) | 2 (0, 7) | 1 (0, 8) | 1 (0, 5) | 3 (0, 9) | 0 (0, 7) | 0 (0, 10) | 2 (0, 10) | 0 (0, 10) |
| 21 Days Post Vaccination 1 | ||||||||||
| n | 102 | 71 | 93 | 68 | 101 | 72 | 50 | 35 | 49 | 31 |
| GMT (95% CI) | 7.4 (6.6, 8.4) | 6.0 (5.4, 6.7) | 7.2 (6.4, 8.0) | 6.6 (5.6, 7.7) | 7.6 (6.7, 8.6) | 7.5 (6.2, 9.0) | 5.6 (5.1, 6.2) | 5.4 (5.0, 5.7) | 5.4 (5.1, 5.8) | 5.8 (4.9, 6.9) |
| Titer ≥40 – % (95% CI) | 4 (1, 10) | 3 (0, 10) | 5 (2, 12) | 4 (1, 12) | 5 (2, 11) | 8 (3, 17) | 2 (0, 11) | 0 (0, 10) | 0 (0, 7) | 3 (0, 17) |
| 7 Days Post Vaccination 2 | ||||||||||
| n | 99 | 69 | 84 | 62 | 95 | 68 | 49 | 33 | 47 | 30 |
| GMT (95% CI) | 56.6 (43.8, 73.1) | 20.2 (15.0, 27.2) | 63.2 (50.6, 79.1) | 28.3 (19.8, 40.3) | 74.6 (59.9, 92.9) | 24.9 (18.5, 33.5) | 5.4 (5.2, 5.7) | 5.4 (5.1, 5.7) | 7.6 (6.4, 9.1) | 5.6 (4.7, 6.6) |
| Titer ≥40 – % (95% CI) | 70 (60, 79) | 39 (28, 52) | 76 (66, 85) | 50 (37, 63) | 79 (69, 87) | 46 (33, 58) | 0 (0, 7) | 0 (0, 11) | 6 (1, 18) | 3 (0, 17) |
| 21 Days Post Vaccination 2 | ||||||||||
| n | 101 | 67 | 85 | 63 | 99 | 67 | 49 | 33 | 43 | 31 |
| GMT (95% CI) | 49.5 (39.1, 62.6) | 23.0 (16.9, 31.2) | 53.0 (42.9, 65.4) | 27.1 (19.9, 36.9) | 59.4 (48.1, 73.3) | 28.4 (21.2, 38.1) | 5.6 (5.3, 5.9) | 5.5 (5.1, 5.9) | 6.9 (5.9, 8.1) | 5.7 (4.8, 6.7) |
| Titer ≥40 – % (95% CI) | 69 (59, 78) | 45 (33, 57) | 71 (60, 80) | 51 (38, 64) | 76 (66, 84) | 49 (37, 62) | 0 (0, 7) | 0 (0, 11) | 2 (0, 12) | 3 (0, 17) |
| 180 Days Post Vaccination 2 | ||||||||||
| n | 95 | 66 | 82 | 61 | 96 | 64 | 48 | 32 | 43 | 30 |
| GMT (95% CI) | 11.1 (9.4, 13.1) | 8.1 (7.0, 9.5) | 12.1 (10.1, 14.4) | 8.5 (7.2, 10.1) | 16.0 (13.4, 19.2) | 12.4 (9.7, 15.7) | 6.2 (5.2, 7.3) | 5.6 (5.3, 6.0) | 5.6 (5.2, 6.1) | 5.5 (5.0, 6.0) |
| Titer ≥40 – % (95% CI) | 17 (10, 26) | 5 (1, 13) | 18 (11, 28) | 7 (2, 16) | 24 (16, 34) | 19 (10, 30) | 4 (1, 14) | 0 (0, 11) | 0 (0, 8) | 0 (0, 12) |
| Day 1 (Pre-Vaccination 1) | Microneutralization Antibody Responses | |||||||||
| n | 106 | 75 | 97 | 71 | 104 | 74 | 51 | 36 | 54 | 34 |
| GMT (95% CI) | 5.1 (5.0, 5.2) | 5.4 (5.1, 5.7) | 5.2 (5.0, 5.4) | 5.4 (5.2, 5.7) | 5.1 (5.0, 5.2) | 5.6 (5.3, 6.0) | 5.1 (5.0, 5.2) | 5.7 (5.1, 6.4) | 5.3 (5.0, 5.6) | 5.8 (4.8, 6.9) |
| Titer ≥40 – % (95% CI) | 0 (0, 3) | 0 (0, 5) | 1 (0, 6) | 0 (0, 5) | 0 (0, 3) | 0 (0, 5) | 0 (0, 7) | 0 (0, 10) | 0 (0, 7) | 3 (0, 15) |
| 7 Days Post Vaccination 1 | ||||||||||
| n | 106 | 75 | 96 | 69 | 104 | 74 | 51 | 36 | 54 | 34 |
| GMT (95% CI) | 5.7 (5.4, 6.1) | 5.5 (5.2, 5.8) | 6.2 (5.7, 6.9) | 6.2 (5.3, 7.4) | 6.4 (6.0, 7.0) | 6.5 (5.7, 7.4) | 5.2 (5.0, 5.5) | 5.9 (5.2, 6.7) | 6.3 (5.5, 7.3) | 5.5 (5.1, 5.9) |
| Titer ≥40 – % (95% CI) | 0 (0, 3) | 0 (0, 5) | 3 (1, 9) | 1 (0, 8) | 0 (0, 3) | 3 (0, 9) | 0 (0, 7) | 0 (0, 10) | 4 (0, 13) | 0 (0, 10) |
| 21 Days Post Vaccination 1 | ||||||||||
| n | 102 | 71 | 93 | 67 | 101 | 72 | 50 | 35 | 49 | 31 |
| GMT (95% CI) | 9.5 (8.3, 10.8) | 7.1 (6.4, 7.9) | 9.5 (8.2, 10.8) | 7.7 (6.4, 9.3) | 10.4 (9.2, 11.7) | 8.7 (7.1, 10.6) | 5.3 (5.1, 5.5) | 5.5 (5.1, 6.0) | 7.0 (5.9, 8.2) | 5.7 (5.2, 6.3) |
| Titer ≥40 – % (95% CI) | 3 (1, 8) | 1 (0, 8) | 5 (2, 12) | 3 (0, 10) | 6 (2, 12) | 6 (2, 14) | 0 (0, 7) | 0 (0, 10) | 2 (0, 11) | 0 (0, 11) |
| 7 Days Post Vaccination 2 | ||||||||||
| N | 100 | 69 | 84 | 62 | 93 | 68 | 49 | 33 | 47 | 30 |
| GMT (95% CI) | 64.1 (51.2, 80.2) | 25.1 (19.8, 31.8) | 74.9 (63.0, 89.0) | 35.0 (25.7, 47.6) | 80.9 (66.0, 99.1) | 33.3 (25.8, 42.9) | 6.2 (5.5, 6.9) | 5.9 (5.2, 6.7) | 9.9 (7.8, 12.6) | 5.9 (5.3, 6.5) |
| Titer ≥40 – % (95% CI) | 76 (66, 84) | 41 (29, 53) | 85 (75, 91) | 52 (39, 65) | 82 (72, 89) | 51 (39, 64) | 0 (0, 7) | 0 (0, 11) | 11 (4, 23) | 0 (0, 12) |
| 21 Days Post Vaccination 2 | ||||||||||
| N | 100 | 68 | 86 | 63 | 99 | 67 | 49 | 33 | 43 | 31 |
| GMT (95% CI) | 65.4 (53.2, 80.5) | 30.5 (24.0, 38.8) | 70.0 (59.2, 82.9) | 36.2 (27.7, 47.5) | 70.5 (59.3, 83.9) | 38.6 (30.1, 49.4) | 5.9 (5.4, 6.5) | 6.6 (5.5, 7.8) | 9.2 (7.3, 11.7) | 6.1 (5.5, 6.8) |
| Titer ≥40 – % (95% CI) | 76 (66, 84) | 50 (38, 62) | 84 (74, 91) | 51 (38, 64) | 84 (75, 90) | 55 (43, 67) | 0 (0, 7) | 0 (0, 11) | 5 (1, 16) | 0 (0, 11) |
| 180 Days Post Vaccination 2 | ||||||||||
| N | 95 | 66 | 82 | 61 | 96 | 64 | 48 | 32 | 43 | 30 |
| GMT (95% CI) | 16.1 (14.1, 18.3) | 10.3 (9.0, 11.8) | 16.5 (14.3, 19.0) | 10.6 (8.9, 12.6) | 20.9 (18.2, 24.0) | 14.6 (12.0, 17.8) | 5.6 (5.1, 6.1) | 5.6 (5.1, 6.1) | 7.0 (6.0, 8.1) | 5.4 (5.1, 5.6) |
| Titer ≥40 – % (95% CI) | 13 (7, 21) | 0 (0, 5) | 13 (7, 23) | 5 (1, 14) | 26 (18, 36) | 9 (4, 19) | 0 (0, 7) | 0 (0, 11) | 0 (0, 8) | 0 (0, 12) |
N = number of participants in the per protocol group; n = number of participants with available results at each timepoint; GMT = Geometric Mean Titer.
After the second vaccination, all of the adjuvanted groups showed a substantial increase in immunologic endpoints in both the 19–64 and ≥65-year-old age strata, which were noted at 7 days, persisted at 21 days, and declined substantially at 180 days after second vaccination but generally remained above the baseline values (Figures 2 and 3). In contrast, the unadjuvanted groups exhibited little response to the second vaccination.
Figure 2.

A) Hemagglutination inhibition and B) Microneutralization GMT against A/Hong Kong/125/2017 by time point, study group, and age stratum.
Figure 3.


Reverse cumulative distributions of A) Hemagglutination inhibition and B) Microneutralization antibody against A/Hong Kong/125/2017 by time point, study group, and age stratum.
Among the three adjuvanted vaccine groups, the younger age stratum had substantially higher HI and MN responses at 7 and 21 days after the second vaccination than the responses of the older age stratum. The younger age stratum also tended to have higher HI and MN responses at 180 days after the second vaccination when compared with the older age stratum. Among the three adjuvanted groups, and within each age stratum, hemagglutinin antigen content (vaccine dose) was not associated with statistically significant differences in HI or MN antibody GMT or proportion with titer ≥40. The MN GMTs tended to be slightly higher than the HI GMTs, but the patterns of response by study group were similar between the two assays. HI and MN antibody responses were strongly positively correlated (for values at 21 days after the second vaccination for all three study groups and age strata, rho=0.89, p <0.001) (data not shown).
Antibody Responses against Homologous and Heterologous H7N9 Strains in Study Group 3 by Age Subgroups
We evaluated HI and MN antibody responses to homologous and heterologous H7N9 strains among age subgroups (19–34 years, 35–49 years, and 50–64 years) of the 19–64-year-old age stratum as well as among the 19–64- and ≥65-year-old strata (Table 3). In the older age-stratum, there were few participants older than 79 years of age and so we did not further stratify that age-stratum. Results for Study Groups 1, 2, and 3 were similar; for simplicity, only the Group 3 results are presented here.
Table 3.
Hemagglutination inhibition and microneutralization antibody responses in Study Group 3 (15 μg A/H7N9 + AS03A) 21 days after the second vaccination, against the vaccine strain (A/Hong Kong/125/2017) and drifted H7N9 strains (A/Shanghai/2/2013 and A/Guangdong/17SF003/2016), by age-group.
| Age group (years) | |||||
|---|---|---|---|---|---|
| 19–34 | 35–49 | 50–64 | 19–64 | ≥ 65 | |
| N=53 | N=26 | N=20* | N=99* | N=67 | |
| H7N9 strain | Hemagglutination Inhibition Antibody Responses | ||||
| Titer ≥40 – % (95% CI) | |||||
| A/Hong Kong/125/2017 | 87 (72, 93) | 69 (48, 86) | 60 (36, 81) | 76 (66, 84) | 49 (37, 62) |
| A/Shanghai/2/2013 | 60 (46, 74) | 35 (17, 56) | 37 (16, 62) | 49 (39, 59) | 27 (17, 39) |
| A/Guangdong/17SF003/2016 | 55 (40, 68) | 46 (27, 67) | 25 (9, 49) | 46 (36, 57) | 25 (16, 37) |
| GMT (95% CI) | |||||
| A/Hong Kong/125/2017 | 76 (59, 98) | 49 (33, 73) | 40 (22, 74) | 59 (48, 73) | 28 (21, 38) |
| A/Shanghai/2/2013 | 43 (33, 55) | 22 (16, 32) | 24 (13, 44) | 32 (26, 39) | 18 (14, 23) |
| A/Guangdong/17SF003/2016 | 35 (27, 46) | 28 (19, 41) | 16 (9, 28) | 28 (23, 35) | 14 (11, 18) |
| Microneutralization Antibody Responses | |||||
| Titer ≥40 – % (95% CI) | |||||
| A/Hong Kong/125/2017 | 92 (82, 98) | 88 (70, 98) | 55 (32, 77) | 84 (75, 90) | 55 (43, 67) |
| A/Shanghai/2/2013 | 73 (59, 84) | 58 (37, 77) | 55 (32, 77) | 65 (55, 75) | 37 (26, 50) |
| A/Guangdong/17SF003/2016 | 43 (30, 58) | 27 (12, 48) | 10 (1, 32) | 32 (23, 42) | 16 (8, 27) |
| GMT (95% CI) | |||||
| A/Hong Kong/125/2017 | 86 (69, 109) | 62 (47, 82) | 48 (30, 79) | 70 (59, 84) | 39 (30, 49) |
| A/Shanghai/2/2013 | 54 (43, 69) | 34 (25, 47) | 35 (21, 56) | 44 (37, 53) | 28 (22, 35) |
| A/Guangdong/17SF003/2016 | 27 (22, 33) | 20 (14, 29) | 17 (11, 26) | 23 (19, 27) | 14 (11, 17) |
N=19 for 50–65 years and N=98 for 19–64 years for the samples tested against A/Shanghai/2/2013
At 21 days after the second vaccination, HI and MN antibody responses to both the heterologous antigenically-related HPAI A/Guangdong/17SF003/2016-like fifth wave CVV and the antigenically distant A/Shanghai/2/2013 2013 first epidemic wave CVV were substantially lower in magnitude than responses to the homologous A/Hong Kong/125/2017 vaccine strain. With all strains, responses were lower in the ≥65-year-old age stratum compared with the younger group. Among persons less than 65 years of age, responses were highest in the youngest age subgroup (19–34 years) and showed trends for successive decline in the 35–49- and 50–64- year-old age-groups. Within the 19–64- and ≥65 age stratum, HI responses to A/Shanghai/2/2013 and A/Guangdong/17SF003/2016 were similar while the MN responses to A/Guangdong/17SF003/2016 tended to be lower than those to A/Shanghai/2/2013.
We evaluated the correlation between age as a continuous variable in days and HI and MN responses to the A/Hong Kong/125/2017 vaccine strain at 21 days after the second vaccination in Groups 1, 2, and 3 (Figure 4). While there was considerable variability in responses by age, across all age groups there was a trend for steady decline in antibody responses with age, even among younger persons (r=−0.42, p<0.001 for HI responses; r=−0.43, p<0.001 for MN responses).
Figure 4.

Correlation of age as a continuous variable (days) with A) Hemagglutination inhibition and B) Microneutralization log-transformed antibody titers against A/Hong Kong/125/2017 at 21 days after the second vaccination.
We also evaluated the correlation of HI titers at 21 days after the second vaccination, in study groups 1–3 combined, against A/Hong Kong/125/2017 versus A/Guangdong/17SF003/2016 and against A/Hong Kong/125/2017 versus A/Shanghai/2/2013. The correlation across all participants is displayed by study arm and stratum (age 19–64 and age ≥65) (Supplemental Figure) and by age-groups of 19–34, 35–49, 50–64, and 65+ years (Figure 5). In both analyses including the four age-groups, the responses to the vaccine strain were highly correlated with responses to the heterologous strains, as evidenced by the high Spearman’s correlation coefficients. Similarly, the responses to both the homologous and heterologous strains tended to be highest in the youngest age-group (19–34 years) and lowest in the 65+ year age-group. This suggests that the degree of cross-reactivity between antigens may depend both on the characteristics of the antigen as well as the magnitude of the responses to the vaccine antigen as determined by age-groups.
Figure 5.


Correlation of hemagglutination inhibition titers at 21 days after the second vaccination in study groups 1–3 combined against A) A/Hong Kong/125/2017 versus A/Guangdong/17SF003/2016 and B) A/Hong Kong/125/2017 versus A/Shanghai/2/2013 and the pattern of responses among the age-groups of 19–34, 35–49, 50–64, and ≥65 years.
HI Responses at 21 days After the Second Vaccination by Prior Seasonal Influenza Vaccination Status in Study Groups 1–3
Most study participants had previously received the 2016–2017 and/or the 2017–2018 influenza vaccine, which precludes direct comparisons of responses by pattern of prior seasonal vaccination (Table 4). However, in a logistic regression model including study group, age stratum (19–64 years and ≥65 years), BMI (<30 kg/m2 vs. ≥30 kg/m2), sex, and prior receipt of seasonal influenza vaccine (no receipt of either the 2016–2017 or the 2017–2018 vaccine vs. prior receipt of either or both vaccines), older age (OR, 0.42; 95% CI, 0.28, 0.62), and prior receipt of seasonal influenza vaccine (OR, 0.35, 95% CI, 0.18, 0.68) were associated with a lower likelihood of achieving an HI antibody titer ≥40 at 21 days after the second vaccination, while study group (vaccine dose), sex, and BMI were not associated with that endpoint.
Table 4.
Hemagglutination inhibition antibody responses against A/Hong Kong/125/2017 (H7N9) in Study Groups 1, 2, and 3 at 21 days after the second vaccination by age stratum and prior seasonal influenza vaccination status.
| Group 1 3.75 pg A/H7N9 + AS03A (N=181) | Group 2 7.5 pg A/H7N9 + AS03A (N=168) | Group 3 15 pg A/H7N9 + AS03A (N=178) | ||||
|---|---|---|---|---|---|---|
| 19–64 | ≥65 | 19–64 | ≥65 | 19–64 | ≥65 | |
| Did Not Receive 2016–2017 or 2017–2018 Seasonal Influenza Vaccination | ||||||
| n | 18 | 2 | 17 | 6 | 27 | 2 |
| GMT (95% CI) | 59 (31, 111) | 95 (0, undefined) | 72 (49, 107) | 85 (22, 327) | 84 (60, 118) | 20 (0, undefined) |
| Titer ≥40 – % (95% CI) | 72 (47, 90) | 100 (16, 100) | 82 (57, 96) | 83 (36, 100) | 93 (76, 99) | 50 (1, 99) |
| Received 2016–2017 and/or 2017–2018 Seasonal Influenza Vaccination | ||||||
| n | 83 | 65 | 68 | 57 | 71 | 65 |
| GMT (95% CI) | 48 (37, 62) | 22 (16, 30) | 49 (38, 63) | 24 (18, 33) | 52 (40, 67) | 29 (21, 39) |
| Titer ≥40 – % (95% CI) | 69 (58, 78) | 43 (31, 56) | 68 (55, 78) | 47 (34, 61) | 69 (57, 79) | 49 (37, 62) |
N = number of participants in the per protocol group; n = number of participants included in the analysis; GMT = Geometric Mean Titer. Undefined confidence interval indicates an unstable estimate due to small number of participants in the stratum.
Safety and Tolerability of the Vaccine Regimens
Overall, the vaccines were well tolerated (Figure 6). Solicited systemic and local AEs tended to be more frequent in the adjuvanted vaccine groups. In a multivariable logistic regression model including age (19–64 years and ≥65 years), sex, study group, and interaction terms for age and study group, participants who received unadjuvanted vaccine (Groups 4 and 5) were significantly less likely than those who received adjuvanted vaccine to report any solicited systemic reaction after any vaccination (Group 4 vs the Group 1 reference group, odds ratio [OR] 0.16, 95% CI, 0.06–0.43) (Group 5 vs Group 1, OR 0.28, 95% CI, 0.11–0.68) or any solicited local reaction (Group 4 vs Group 1, OR 0.13, 95% CI, 0.04–0.38) (Group 5 vs Group 1, OR 0.14, 95% CI, 0.16–0.41). In those models, age ≥65 years was also associated with a reduced risk of any solicited systemic reaction (OR, 0.45, 95% CI, 0.25–0.82) and any solicited local reaction (OR 0.38, 95% CI, 0.16–0.93), sex was not associated with risk in either model, and, within the adjuvanted and unadjuvanted groups, antigen content was also not associated with risk in either model.
Figure 6.


Percentage of participants experiencing solicited systemic and local AEs after any study vaccination, by maximum severity and study group.
Thirty-two SAEs, including three deaths, all due to cancer, were reported across all study groups; none were considered to be related to the study vaccine (Supplementary Table). Two PIMMCs, inflammatory arthritis, with onset 56 days after the second vaccination, and Graves’ disease, identified by laboratory tests obtained 152 days after the second vaccination, were reported in members of study group 1. An alternate etiology was not identified for either AE and both were therefore considered to be related to the study product, and were also considered MAAEs and NOCMCs. Four other MAAEs were considered related to study product (tooth infection noted three days after vaccination [Group 1], intermittent fatigue and intermittent malaise [both MAAEs in the same participant in Group 2], and left sided neck soreness [Group 3], all moderate in severity). No other NOCMCs were considered related to study product. Clinical laboratory AEs were infrequent and were nearly exclusively mild in severity.
DISCUSSION
In this trial we found that a single dose of the inactivated H7N9 fifth wave vaccine, with or without adjuvant, is poorly immunogenic, as is a two-dose schedule of unadjuvanted vaccine. We found the greatest responses after two doses of AS03A-adjuvanted vaccine, which induced an HI antibody titer ≥40 in 69% to 76% of participants under 65 years of age and 45% to 51% of those ≥65 years of age at 21 days after the second vaccination, with marked waning of those responses at 180 days after the second vaccination. The AS03A adjuvant was also shown to permit dose sparing of HA antigen, with no significant differences in responses after the second vaccination across the range of antigen dose levels, from 3.75 μg to 15 μg. These findings are consistent with those of the previous trial of the 2013 influenza A/Shanghai/2/2013 H7N9 vaccine given with and without AS03 adjuvant, which was also conducted in the VTEU network, and with other evaluations of H7N9 vaccines.19, 22, 23, 25–28 Together, these results suggest that, in the event of a threat from circulating H7N9 virus, adjuvanted vaccine formulated with 3.75 μg of antigen would allow production of a larger number of doses if the antigen supply is constrained.
Antibody responses in the adjuvanted groups diminished with increasing age, with the lowest responses in the ≥65-year-old age-group. However, even among those less than 65 years of age there was evidence for a reduction in HI and MN responses with age, which has also been previously reported.19, 23 Evaluation of the correlation of age as a continuous variable (in days) with log-transformed HI and MN titers at 21 days after the second vaccination indicates that the relationship between age and response is relatively linear and moderately negatively correlated. This suggests that, to the extent that antibody responses correlate with vaccine effectiveness, estimates of antibody responses based on broad age-groups, such as 19 through 64 years, may overestimate effectiveness among persons in the older end of that grouping and underestimate effectiveness in younger persons. In a logistic regression model, age ≥65 years and prior receipt of seasonal influenza vaccine were independently associated with a lower likelihood of achieving an HI antibody titer ≥40, consistent with previous evaluations.19, 23
We also evaluated heterologous responses to the antigenically related HPAI strain A/Guangdong/17SF003/2016 (H7N9), which co-circulated in China with the A/Hong Kong/125/2017 strain in the fifth wave, and to the antigenically distant first wave A/Shanghai/2/2013 CVV strain, and found patterns of responses that were similar to those against the homologous A/Hong Kong/125/2017 vaccine strain but consistently lower in magnitude. This suggests that, in the event of sustained human-to-human transmission of an influenza/A H7N9 strain, a vaccine strain that is well matched to the circulating strain would offer the most robust likelihood of protection. We also found that, among persons who received adjuvanted vaccine, those in the youngest age-group of 19–34 years tended to have had the highest responses to the homologous vaccine antigen as well as to the heterologous antigens, while those 65 years of age and older tended to have the lowest responses, suggesting that cross-protection against heterologous H7N9 strains may also vary by age, and immune history.
Interestingly, an evaluation of a recombinant AS03 adjuvanted influenza vaccine derived from the A/Guangdong/17SF003/2016 fifth-wave CVV elicited strong cross-reactive HI responses to both the antigenically related A/Hong Kong/125/2017 strain as well as to the antigenically distant A/Shanghai/2/2013 strain in healthy adults less than 50 years of age, with an HI antibody titer ≥40 against A/Shanghai/2/2013 in 82% of those participants.28 In contrast, we found an HI antibody titer ≥40 against A/Shanghai/2/2013 in only 60% of persons 19–34 years of age and 35% in those 35–49 years of age. The high degree of cross-reactivity induced by the recombinant protein vaccine may be due to the characteristics of that vaccine antigen or possibly to differences in the assays; however, the assays for both trials were conducted under the same qualified protocol at Southern Research (Birmingham, AL).
The adjuvanted vaccine formulations were well tolerated and in a logistic regression analysis persons ≥65 years were less likely to report solicited AEs than younger persons. In another logistic regression analysis, age ≥65 years and prior receipt of seasonal influenza vaccine were independently associated with a lower likelihood of achieving an HI titer ≥40 at 21 days after the second vaccination. The association of prior seasonal influenza vaccination and reduced responses to H7N9 adjuvanted inactivated influenza vaccines has been consistently noted19, 23, 26, 29 and suggests interference from pre-existing immunity. The mechanism(s) of this interference are uncertain but could include cross-reactive antibody binding to conserved stem antigens and/or cellular responses. Importantly, in a study looking at simultaneous versus sequential vaccination, seasonal influenza vaccine was immunogenic whether given with or without H7N9 vaccine.22
This study is subject to limitations. The study was not designed to test any specific null hypothesis but rather it was intended to obtain sufficient data to obtain meaningful estimates of the immune response induced by the various vaccine formulations and to uncover any safety issues that occur at a sufficiently high rate that they might be observed in a study of this size. We did not assess the durability of vaccine-induced responses beyond day 180 after the second vaccination, which may be important in assessing the possible need for booster vaccinations in the event that the avian influenza virus circulate over relatively long durations of time. We also did not assess possible differences in responses with longer intervals between vaccinations nor did we evaluate cellular immune responses, which may contribute to immunogenicity.
The evolving genetic features of A(H7N9) viruses, as well as the changing geographic distribution of human cases, raised concerns about the pandemic potential of fifth-wave circulating A(H7N9) viruses. Among all novel influenza viruses assessed using CDC’s Influenza Risk Assessment Tool through 2019,30 both the 2013 influenza A(H7N9) virus (A/Shanghai/02/2013) and the 2016 YRD lineage influenza A(H7N9) virus (A/Hong Kong/125/2017) were ranked as the viruses with the highest potential pandemic risk (moderate to high).31 This study of AS03A adjuvanted fifth-wave influenza vaccine formulations provides immunogenicity and safety information that may be informative to influenza pandemic preparedness programs.
Supplementary Material
Mild events are those that require minimal or no treatment and do not interfere with daily activities. Moderate events are those that result in a low level of inconvenience or require therapeutic measures and may cause some interference with functioning and daily activities. Severe events are those that interrupt the participant’s usual daily activities.
Temperature values are noted only if ≥38.0°C (lower limit of graded fever) and are reported as mild (38.0°C - 38.4°C), moderate (38.5°C - 38.9°C), or severe (>38.9°C).
Maximal diameter of areas of bruising, erythema, or induration are reported as mild (<20 mm), moderate (20 mm – 50 mm), or severe (>50 mm).
Acknowledgments.
The study was sponsored and designed by the NIH. The sponsor was responsible for study management and the data analysis. All authors were involved in the collection, analysis, or interpretation of the data. The authors vouch for the accuracy and completeness of the data and analyses and for the fidelity of the study to the protocol.
Conflict of Interest Disclosures:
LAJ reports funding support to her institution from Pfizer for the conduct of a clinical trial of an investigational influenza vaccine. NGR is a paid safety consultant for ICON and EMMES, serves on the advisory boards for GSK, Moderna, Sanofi, and Seqirus and her institution received funds for the conduct of research from Sanofi, Lilly, Merck, Quidel, and Pfizer. MJM reported potential competing interests: laboratory research and clinical trials contract funding with Lilly, Pfizer, and Sanofi; personal fees for Scientific Advisory Board service from Merck, Meissa Vaccines, Inc. and Pfizer. EBW has received funding support from Pfizer, Moderna, Sequiris, Najit Technologies Inc, and Clinetic for the conduct of clinical trials and clinical research and has served as an advisor to Vaxcyte and consultant to ILiAD biotechnologies. CAR.’s institution has received funds to conduct clinical research unrelated to this manuscript from BioFire Inc, GSK, MedImmune, Micron, Janssen, Merck, Moderna, Novavax, PaxVax, Pfizer, Regeneron, Sanofi-Pasteur. She is co-inventor of patented RSV vaccine technology unrelated to this manuscript, which has been licensed to Meissa Vaccines, Inc.
Financial support:
This work was supported by federal funds from NIAID, the NIH, and the US Department of Health and Human Services under contracts HHSN272201300019I (Kaiser Washington), HHSN272201300018I (Emory University School of Medicine), HHSN272200800008C (University of Iowa), HHSN272201300022I (University of Maryland), HHSN2722013000017I (Duke University), (Battelle and subcontractor Southern Research, Inc.) and 75N93021C00012 (The EMMES Company, LLC). At the University of Maryland, partial support was also provided by additional funding sources, including the University of Maryland General Clinical Research Center grant M01-RR-016500 from the NCRR, and by NCRR grant K12-RR-023250. At NYU the effort of MJM was also supported by NIH awards AI148574 and 75N93021C00014.
The H7N9 vaccine and AS03 adjuvant were manufactured by Sanofi and GSK, respectively. The work reported herein was also supported in whole or in part with federal funds from the US Department of Health and Human Services; Administration for Strategic Preparedness and Response; BARDA under contracts HHSO100201600004I and HHSO100201600006I. AS03 is a trademark owned by or licensed to the GSK group of companies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclaimer: The opinions, findings, and conclusions in this report are those of the authors and do not necessarily represent the views of the Department of Health and Human Services or its components.
References
- 1.Gao R, Cao B, Hu Y, et al. Human infection with a novel avian-origin influenza A (H7N9) virus. N Engl J Med. May 16 2013;368(20):1888–97. doi: 10.1056/NEJMoa1304459 [DOI] [PubMed] [Google Scholar]
- 2.Li Q, Zhou L, Zhou M, et al. Epidemiology of human infections with avian influenza A(H7N9) virus in China. N Engl J Med. Feb 6 2014;370(6):520–32. doi: 10.1056/NEJMoa1304617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kile JC, Ren R, Liu L, et al. Update: Increase in Human Infections with Novel Asian Lineage Avian Influenza A(H7N9) Viruses During the Fifth Epidemic - China, October 1, 2016-August 7, 2017. MMWR Morb Mortal Wkly Rep. Sep 8 2017;66(35):928–932. doi: 10.15585/mmwr.mm6635a2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang X, Jiang H, Wu P, et al. Epidemiology of avian influenza A H7N9 virus in human beings across five epidemics in mainland China, 2013–17: an epidemiological study of laboratory-confirmed case series. Lancet Infect Dis. Aug 2017;17(8):822–832. doi: 10.1016/S1473-3099(17)30323-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Qi X, Qian YH, Bao CJ, et al. Probable person to person transmission of novel avian influenza A (H7N9) virus in Eastern China, 2013: epidemiological investigation. BMJ. Aug 6 2013;347:f4752. doi: 10.1136/bmj.f4752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang J, Su N, Dong Z, et al. The fifth influenza A(H7N9) epidemic: A family cluster of infection in Suzhou city of China, 2016. Int J Infect Dis. Sep 2018;74:128–135. doi: 10.1016/j.ijid.2018.04.4322 [DOI] [PubMed] [Google Scholar]
- 7.Wang X, Wu P, Pei Y, et al. Assessment of Human-to-Human Transmissibility of Avian Influenza A(H7N9) Virus Across 5 Waves by Analyzing Clusters of Case Patients in Mainland China, 2013–2017. Clin Infect Dis. Feb 1 2019;68(4):623–631. doi: 10.1093/cid/ciy541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu B, Havers FP, Zhou L, et al. Clusters of Human Infections With Avian Influenza A(H7N9) Virus in China, March 2013 to June 2015. J Infect Dis. Sep 15 2017;216(suppl_4):S548–S554. doi: 10.1093/infdis/jix098 [DOI] [PubMed] [Google Scholar]
- 9.Zhou L, Chen E, Bao C, et al. Clusters of Human Infection and Human-to-Human Transmission of Avian Influenza A(H7N9) Virus, 2013–2017. Emerg Infect Dis. Feb 2018;24(2)doi: 10.3201/eid2402.171565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen H, Liu S, Liu J, et al. Nosocomial Co-Transmission of Avian Influenza A(H7N9) and A(H1N1)pdm09 Viruses between 2 Patients with Hematologic Disorders. Emerg Infect Dis. Apr 2016;22(4):598–607. doi: 10.3201/eid2204.151561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang Q, Shi J, Deng G, et al. H7N9 influenza viruses are transmissible in ferrets by respiratory droplet. Science. Jul 26 2013;341(6144):410–4. doi: 10.1126/science.1240532 [DOI] [PubMed] [Google Scholar]
- 12.Belser JA, Gustin KM, Pearce MB, et al. Pathogenesis and transmission of avian influenza A (H7N9) virus in ferrets and mice. Nature. Sep 26 2013;501(7468):556–9. doi: 10.1038/nature12391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.World Health Organization. Influenza at the human-animal interface. Accessed 26 December, 2019. https://www.who.int/influenza/human_animal_interface/Influenza_Summary_IRA_HA_interface_09_27_2017.pdf?ua=1
- 14.Wang D, Yang L, Zhu W, et al. Two Outbreak Sources of Influenza A (H7N9) Viruses Have Been Established in China. J Virol. Jun 15 2016;90(12):5561–5573. doi: 10.1128/JVI.03173-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhu W, Zhou J, Li Z, et al. Biological characterisation of the emerged highly pathogenic avian influenza (HPAI) A(H7N9) viruses in humans, in mainland China, 2016 to 2017. Euro Surveill. May 11 2017;22(19)doi: 10.2807/1560-7917.ES.2017.22.19.30533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang L, Zhu W, Li X, et al. Genesis and Spread of Newly Emerged Highly Pathogenic H7N9 Avian Viruses in Mainland China. J Virol. Dec 1 2017;91(23)doi: 10.1128/JVI.01277-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang F, Bi Y, Wang J, et al. Human infections with recently-emerging highly pathogenic H7N9 avian influenza virus in China. J Infect. Jul 2017;75(1):71–75. doi: 10.1016/j.jinf.2017.04.001 [DOI] [PubMed] [Google Scholar]
- 18.Ke C, Mok CKP, Zhu W, et al. Human Infection with Highly Pathogenic Avian Influenza A(H7N9) Virus, China. Emerg Infect Dis. Jul 2017;23(8):1332–1340. doi: 10.3201/eid2308.170600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jackson LA, Campbell JD, Frey SE, et al. Effect of Varying Doses of a Monovalent H7N9 Influenza Vaccine With and Without AS03 and MF59 Adjuvants on Immune Response: A Randomized Clinical Trial. JAMA. Jul 21 2015;314(3):237–46. doi: 10.1001/jama.2015.7916 [DOI] [PubMed] [Google Scholar]
- 20.Zhong W, Levine MZ. Stockpiled Avian Influenza A(H7N9) Vaccines Induce Robust, Nonneutralizing Functional Antibodies Against Antigenically Drifted Fifth-Wave A(H7N9) Viruses. J Infect Dis. Sep 13 2019;220(8):1276–1280. doi: 10.1093/infdis/jiz295 [DOI] [PubMed] [Google Scholar]
- 21.World Health Organization. Summary of status of development and availability of avian influenza A(H7N9) cadidate vaccine viruses and potency testing reagents.. World Health Organization. Accessed 26 December, 2019. https://www.who.int/influenza/vaccines/virus/candidates_reagents/summary_a_h7n9_cvv_20170518.pdf?us=1 [Google Scholar]
- 22.Ortiz JR, Spearman PW, Goepfert PA, et al. Safety and immunogenicity of monovalent H7N9 influenza vaccine with AS03 adjuvant given sequentially or simultaneously with a seasonal influenza vaccine: A randomized clinical trial. Vaccine. May 20 2022;40(23):3253–3262. doi: 10.1016/j.vaccine.2022.03.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mulligan MJ, Bernstein DI, Winokur P, et al. Serological responses to an avian influenza A/H7N9 vaccine mixed at the point-of-use with MF59 adjuvant: a randomized clinical trial. JAMA. Oct 8 2014;312(14):1409–19. doi: 10.1001/jama.2014.12854 [DOI] [PubMed] [Google Scholar]
- 24.Keitel WA, Dekker CL, Mink C, et al. Safety and immunogenicity of inactivated, Vero cell culture-derived whole virus influenza A/H5N1 vaccine given alone or with aluminum hydroxide adjuvant in healthy adults. Vaccine. Nov 5 2009;27(47):6642–8. doi: 10.1016/j.vaccine.2009.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Madan A, Segall N, Ferguson M, et al. Immunogenicity and Safety of an AS03-Adjuvanted H7N9 Pandemic Influenza Vaccine in a Randomized Trial in Healthy Adults. J Infect Dis. Dec 1 2016;214(11):1717–1727. doi: 10.1093/infdis/jiw414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vanni T, Thome BC, Sparrow E, et al. Dose-sparing effect of two adjuvant formulations with a pandemic influenza A/H7N9 vaccine: A randomized, double-blind, placebo-controlled, phase 1 clinical trial. PLoS One. 2022;17(10):e0274943. doi: 10.1371/journal.pone.0274943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fries LF, Smith GE, Glenn GM. A recombinant viruslike particle influenza A (H7N9) vaccine. N Engl J Med. Dec 26 2013;369(26):2564–6. doi: 10.1056/NEJMc1313186 [DOI] [PubMed] [Google Scholar]
- 28.Oshansky CM, King J, Lu D, et al. Adjuvanted recombinant hemagglutinin H7 vaccine to highly pathogenic influenza A(H7N9) elicits high and sustained antibody responses in healthy adults. NPJ Vaccines. Mar 19 2021;6(1):41. doi: 10.1038/s41541-021-00287-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Winokur P, El Sahly HM, Mulligan MJ, et al. Immunogenicity and safety of different dose schedules and antigen doses of an MF59-adjuvanted H7N9 vaccine in healthy adults aged 65 years and older. Vaccine. Feb 22 2021;39(8):1339–1348. doi: 10.1016/j.vaccine.2020.11.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Centers for Disease Control and Prevention. Influenza risk assessment tool (IRAT). Accessed 26 December, 2019. https://www.cdc.gov/flu/pandemic-resources/national-strategy/risk-assessment.htm
- 31.Centers for Disease Control and Prevention. Summary of Influenza Risk Assessment Tool (IRAT) Results. US Department of Health and Human Services, CDC. Accessed December 27, 2019. https://www.cdc.gov/flu/pandemic-resources/monitoring/irat-virus-summaries.htm#H7N9_hongkong [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


