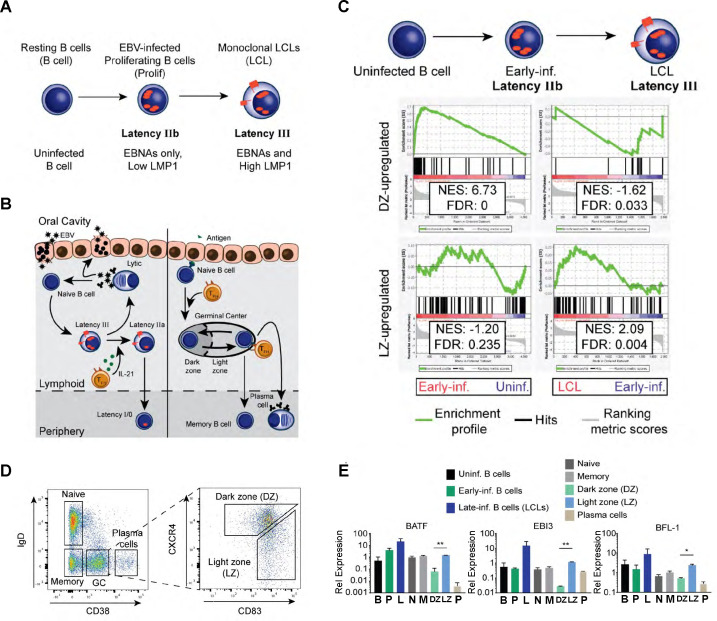
Fig 1.
High BFL-1 expression typifies GC LZ B cells and EBV-immortalized LCLs. (A) Schematic of EBV infection of human B cells in vitro including transitions through latency IIb (EBNAs only) to latency III (EBNAs and LMPs). (B) Schematic comparing EBV infection in vivo with B-cell maturation in the oral cavity. Left, upon saliva transmission, EBV viral particles first infect an epithelial cell to amplify the initial viral load and increase the likelihood of infecting a naïve B cell in the lymphoid tissue. EBV infection activates the infected naïve B cell and stimulates it to proliferate through the expression of viral and host genes. First, EBV-infected B cells express the latency IIb gene expression program, in which all EBNAs are expressed. Then, the EBNAs activate the expression of the LMP, LMP1, and LMP2A, in latency III. IL-21 secretion by T follicular helper cells (TFH) in the lymphoid follicle induces the transition to latency IIa by silencing the expression of the EBNAs (33). Eventually, EBV-infected B cells attenuate viral gene expression in latency 0 but periodically express EBNA1 in latency I to maintain the viral genome in latently infected B cells. Plasma cell differentiation of EBV-infected B cells leads to lytic reactivation and production of infectious virion particles. Right, affinity maturation of B-cell antigen receptors is initiated when antigen encounter activates and stimulates naïve B cells to proliferate and form the GC reaction. In the GC DZ, hyperproliferating B cells undergo class-switch recombination and somatic hypermutation and transit to the GC LZ where they compete for antigen signaling and CD40 ligation from cognate TFH. Surviving B cells exit as plasma cells or as memory B cells. (C) Gene set enrichment analysis (GSEA) comparing differential gene expression between GC B cells and EBV-infected B cells in vitro. (D) Flow cytometry plots of sorting strategy of CD19+ B cell subsets from tonsillar lymphocytes. (E) qPCR comparing gene expression of BATF, EBI3, and BFL-1 from in vitro EBV-infected B cells and sorted tonsillar B cells. Data are representative of two experiments. Mean and SEM are plotted from two experiments and normalized to SETDB1 and uninfected B cells. Significance was determined by unpaired t-test between DZ and LZ B cells. *P < 0.05, **P < 0.01.