Fig 2.
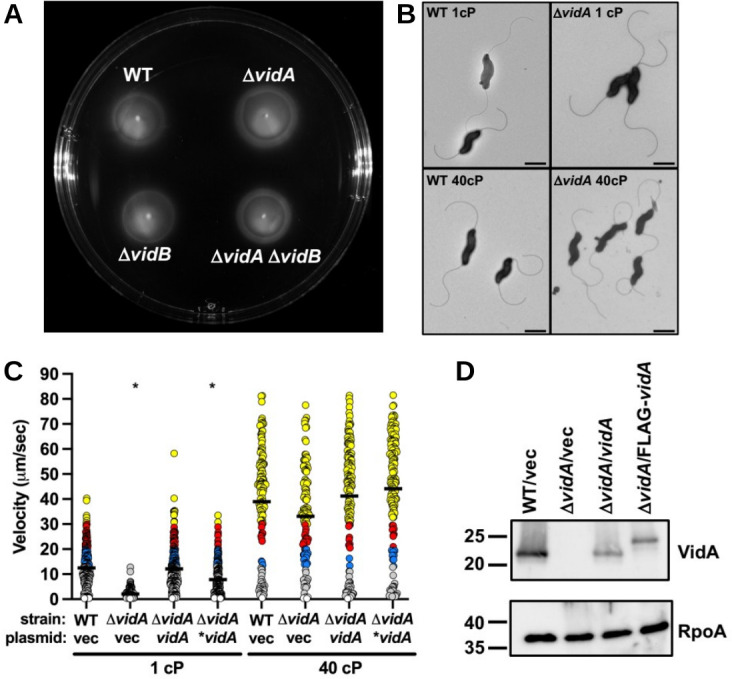
Flagellation and motility phenotypes of C. jejuni ΔvidA. (A) Motility assay in semi-solid motility agar. Motility assays of WT C. jejuni and isogenic ΔvidA and ΔvidB mutants were performed by stabbing cultures of similar optical densities into MH motility medium containing 0.4% agar. Plates were incubated in microaerobic conditions at 37°C for 30 h. (B) Electron micrographs of WT C. jejuni and C. jejuni ΔvidA grown in media of different viscosities. After overnight growth in MH broth alone (1 cP) or MH broth with methylcellulose (40 cP), strains were visualized by transmission electron microscopy. Bar = 1 µm. (C) Swimming velocities of C. jejuni ΔvidA upon in trans complementation with WT vidA. Swimming velocity of WT with vector (vec) alone, ΔvidA with vector alone, ΔvidA with vector encoding WT vidA, and ΔvidA with vector encoding an N-terminal FLAG-tagged VidA (*vidA) after growth for 24 h in MH broth alone (1 cP) or MH broth with methylcellulose to achieve a viscosity of 40 cP. Swimming velocities of individual cells (n > 100) were measured by video tracking under dark-field microscopy. Assays were performed in triplicate and combined. Circles represent individual cells with velocities of <1 µm/s (white), 1–13 μm/s (gray), 13–20 μm/s (blue), 20–30 μm/s (red), and >30 µm/s (yellow). The statistical significance of the difference in swimming velocities between the WT strain with the vector alone and other strains at both 1 and 40 cP was calculated using one-way ANOVA followed by Tukey’s multiple comparisons test. *P < 0.05. (D) Immunoblot analysis of VidA in whole-cell lysates of WT C. jejuni or C. jejuni ΔvidA containing vector (vec) alone or vectors encoding WT vidA or N-terminal FLAG-tagged VidA (*vidA). Specific antiserum to VidA was used. Detection of RpoA serves as a control to ensure equal loading of proteins across strains.
