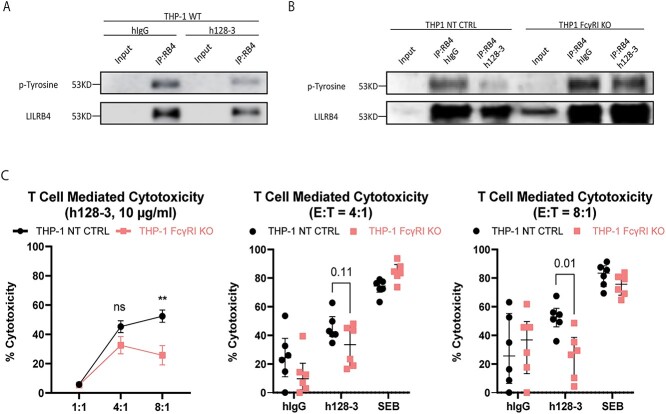
Figure 5.
FcγRI crosslinking enhances the LILRB4 blocking function of h128-3, leading to improved T-cell-mediated cytotoxicity of FcγR high monocytic AML. (A, B) THP-1 WT (A), NT CTRL and FcγRI KO (B) cells (1 × 107) were serum-starved for 18 h and then incubated with PBS, hIgG or h128-3 (10 μg/ml) for 1 h at 37°C. The cells were then seeded on non-treated tissue culture plates with pre-bound ApoE2 (5 μg/ml, Peprotech) and anti-HLA-DR antibody (5 μg/ml, L243, Biolegend) for 15 min at 37°C to stimulate rapid LILRB4 activation before lysis at 4°C in the presence of protease and phosphatase inhibitors. LILRB4 was immunoprecipitated from the lysed cells overnight at 4°C using a high-affinity anti-LILRB4 antibody (R8), and western blot was run on the immunoprecipitated LILRB4. The membrane was probed overnight at 4°C with anti-pTyrosine antibody (4G10, Cell Signaling), stripped with mild stripping buffer and then re-probed with anti-LILRB4 (R8) for 1 h at RT for loading comparison. (C) To determine T-cell-mediated cytotoxicity induced by h128-3, CD3+ T cells were first isolated from healthy donor PBMCs by negative selection and expanded for 48 h at 37°C in medium enriched with ImmunoCult Human CD3/CD28 T-Cell Activator (25 μl/106 cells/ml, Stemcell Technologies), rIL-7 and rIL-15 (10 ng/ml, Peprotech). GFP+ THP-1 NT CTRL and FcγRI KO cells were then seeded in 96 well U-bottom plates in normal R10 media (untreated) or R10 supplemented with isotype control hIgG (10 μg/ml), h128-3 (10 μg/ml) or Staphylococcus enterotoxin B (2 μg/ml) for 15 min at 37°C. Expanded T cells were then seeded in co-culture with the monocytic AML cells at E:T ratios of 1:1, 4:1 and 8:1 for 24 h at 37°C. The co-cultured cells were washed with 2% BSA/PBS and live/dead-stained with DAPI before analysis by flow cytometry. Live target THP-1 cells (GFP+DAPI−) were gated and antibody- or toxin-mediated T-cell cytotoxicity of NT CTRL or FcγRI KO THP-1 cells was calculated relative to T-cell cytotoxicity of untreated NT CTRL or FcγRI KO THP-1 cells, respectively.