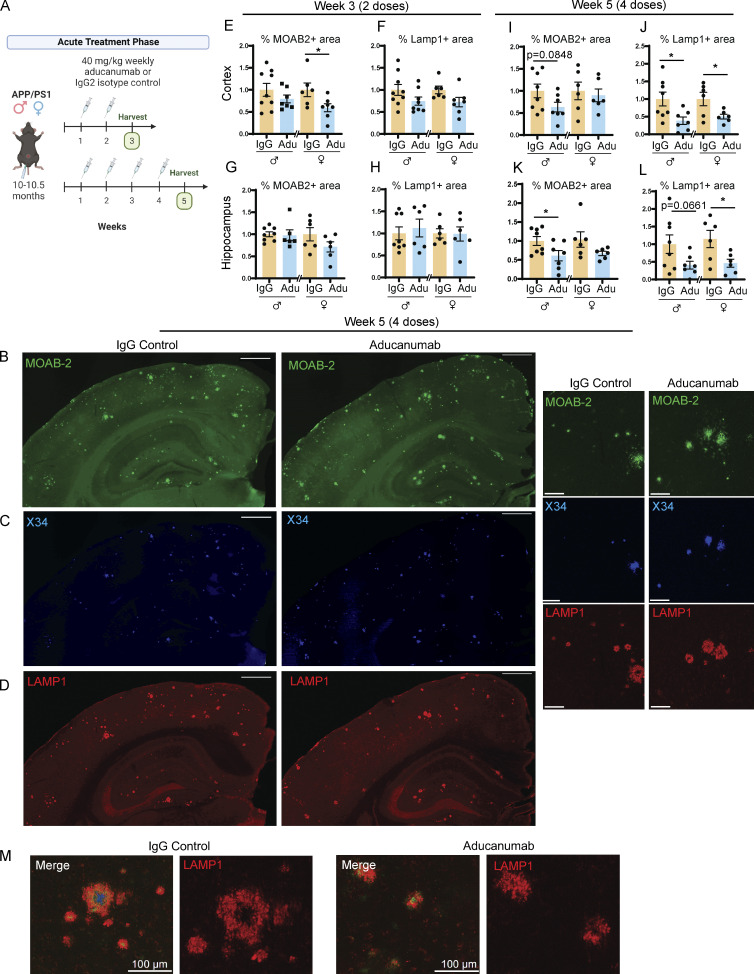
Figure 1.
Aducanumab modestly reduces Aβ and neuritic dystrophy in APP/PS1 mice during acute treatment phases. (A) Timeline of the acute treatment phase of the study. Male and female 10- to 10.5-mo-old APP/PS1 mice received either two or four doses of aducanumab or IgG control (40 mg/kg, IP injection) and were harvested for analysis 1 wk after the final dose. (B–D) Representative images of (B) total Aβ marked by MOAB-2, (C) X34+ fibrillar plaques, and (D) neuritic dystrophy marked by LAMP1, in male mice treated with four doses of aducanumab (Adu) or IgG control. (E and F) Quantification of percent of the tissue covered by MOAB-2+ (E) and LAMP1+ staining in the cortex (F), following two doses of aducanumab. (G and H) Quantification of percent of the tissue covered by MOAB-2+ (G) and LAMP1+ staining in the hippocampus (H), following two doses of aducanumab. (I and J) Quantification of the percent of tissue covered by MOAB-2+ (I) and LAMP1 staining in the cortex (J), following four doses of aducanumab. (K and L) Quantification of percent of tissue covered by MOAB-2+ (K) and LAMP1 staining in the hippocampus (L), following four doses of aducanumab. (M) LAMP1+ neuritic dystrophy surrounding amyloid plaques in IgG control and aducanumab-treated mice. Scale bars in B–D = 500 µm for whole tissue view, 100 µm for insets; scale bars in M = 100 µm. Data in E–L expressed as fold change relative to IgG control of the same sex. *P < 0.05; **P < 0.01, Student’s t test for normally distributed samples with no significant difference in variance (E; F; G females; H females; I; J males; K males; L females), Welch’s t test for normally distributed samples with differences in variances (J females; K females; L males), Mann–Whitney test for non-normally distributed samples (G males; H males), with males and females analyzed separately. N = 6–9 mice/sex/treatment.