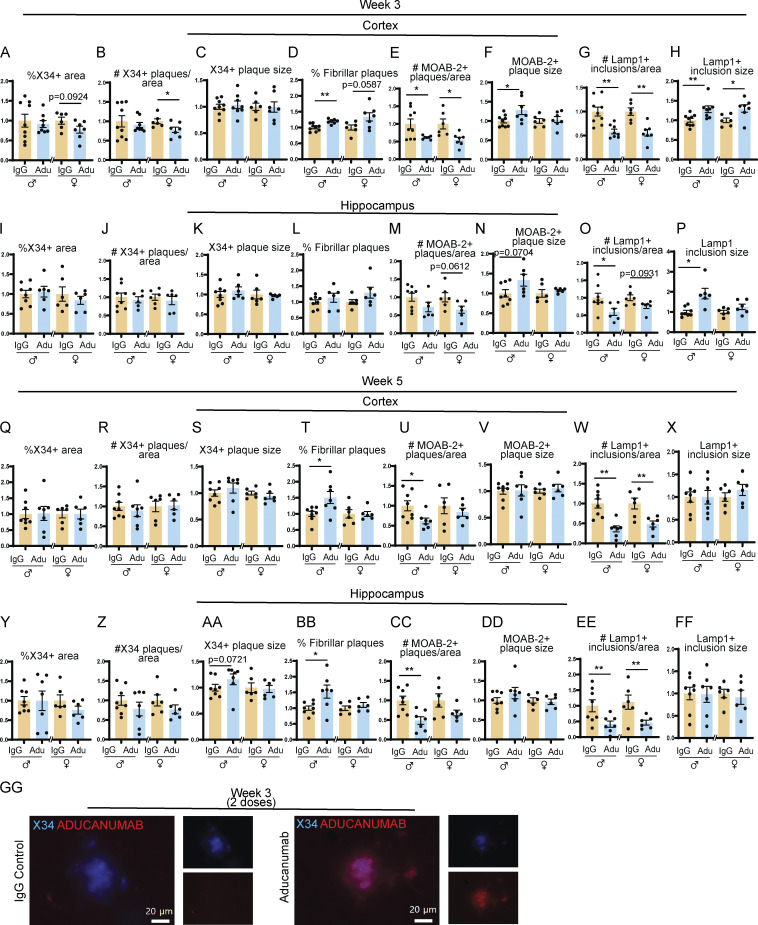
Figure S1.
Aducanumab modestly reduces total Aβ after two and four doses. (A–H) Quantification of plaques and neuritic dystrophy in the cortex after two weekly doses of IgG or aducanumab (Adu). (A) Percent of the tissue covered by X34+ staining (A), number of X34+ plaques per area (B), average X34+ plaque size (C), percent of X34+ fibrillar plaques (D), number of MOAB-2+ plaques per area (E), average MOAB-2+ plaque size (F), number of LAMP1+ inclusions per area (G), average LAMP1+ inclusion size (H). (I–P) The same as A–H, but in the hippocampus, after two weekly doses of IgG or aducanumab. (Q–X) The same as A–H, but in the cortex, after four weekly doses of IgG or aducanumab. (Y–FF) The same as A–H, but in the hippocampus, after four weekly doses of IgG or aducanumab. (GG) Staining of X34+ plaques with Cy3 mouse IgG secondary antibody to detect aducanumab in the acute treatment phase. *P < 0.05; **P < 0.01, Student’s t test for normally distributed samples with no significant difference in variance (A; B females; C; D; E females; F; G; H females; I; J males; K males; L; M; N males; O males; P females; Q; R males; S females; T females; U–Z; AA females; BB females; CC; DD; EE males; FF), Welch’s t test for normally distributed samples with differences in variances (E males; K females; N females; P males; BB males; EE females), Mann–Whitney test for non-normally distributed samples (B males; H males; J females; O females; R females; S males; T males; AA males), with males and females analyzed separately. N = 6–9 mice/sex/treatment.