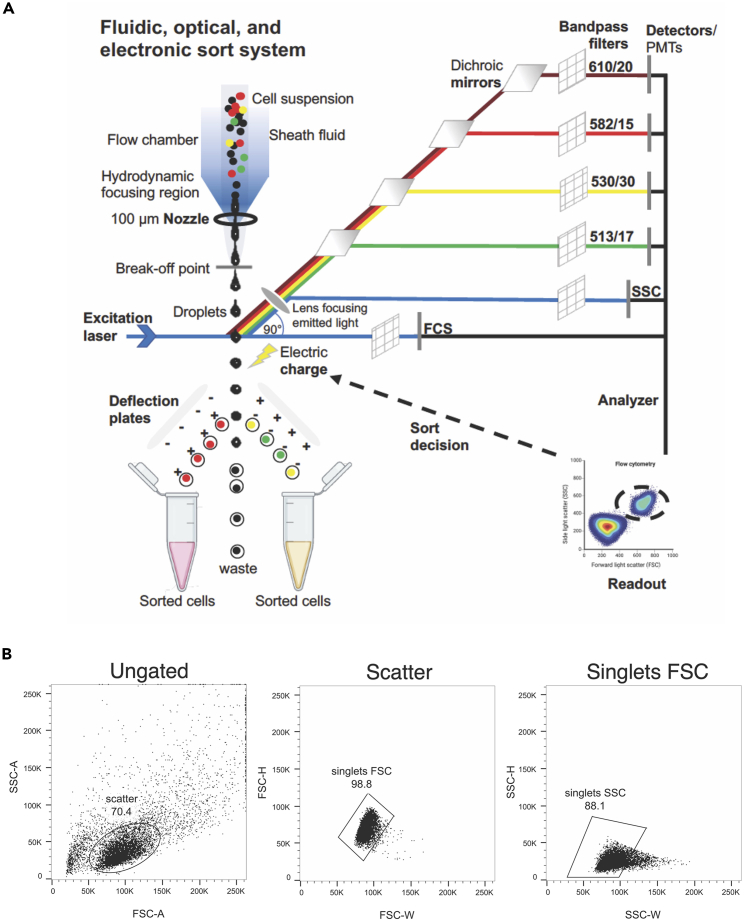
Figure 4.
Principle of flow cytometry for sorting early neurons
(A) The cell suspension containing transfected and non-transfected cells passes through a 100 μm nozzle and undergoes hydrodynamic focusing within the flow cell due to pressure differences between the sheath fluid and the cell suspension. After separating the stream into single droplets containing one or more cells, the excitation laser interrogates each droplet. Depending on the size (forward scatter, FSC), granularity (side scatter, SSC), and wavelength of the emitted light, a specific sorting decision is formulated by the user. Mirrors deflect the incoming laser beam and filters pass a specific wavelength range to the respective detectors. The fluorophores used here required bandpass filters of 610/20 (dsRed2), 582/15 (tDimer), 530/30 (Venus), and 513/17 (eGFP). The resulting conversion of light to an electrical charge determines the deflection of cells into their respective sorting tubes. Non-transfected cells are not charged and go to the waste container. PTM = Photomultiplier tube.
(B) Each sort decision begins with identifying the cell population of interest by plotting SSC-area (SSC-A) against FSC-A to compare the size and granularity of the scatter. Second, duplicates in this scatter were excluded by matching the width (W) against the height (H) of the FSC. These gated FSC singlets served as input for the detected cells in the SSC-H versus SSC-W plot. The resultingSSC singlet cell populationserved as the final input for differential fluorescence detection every time the respective transfected cells were sorted (seeFigures 5B and 6B) and was employed for sorting the constructs used here.