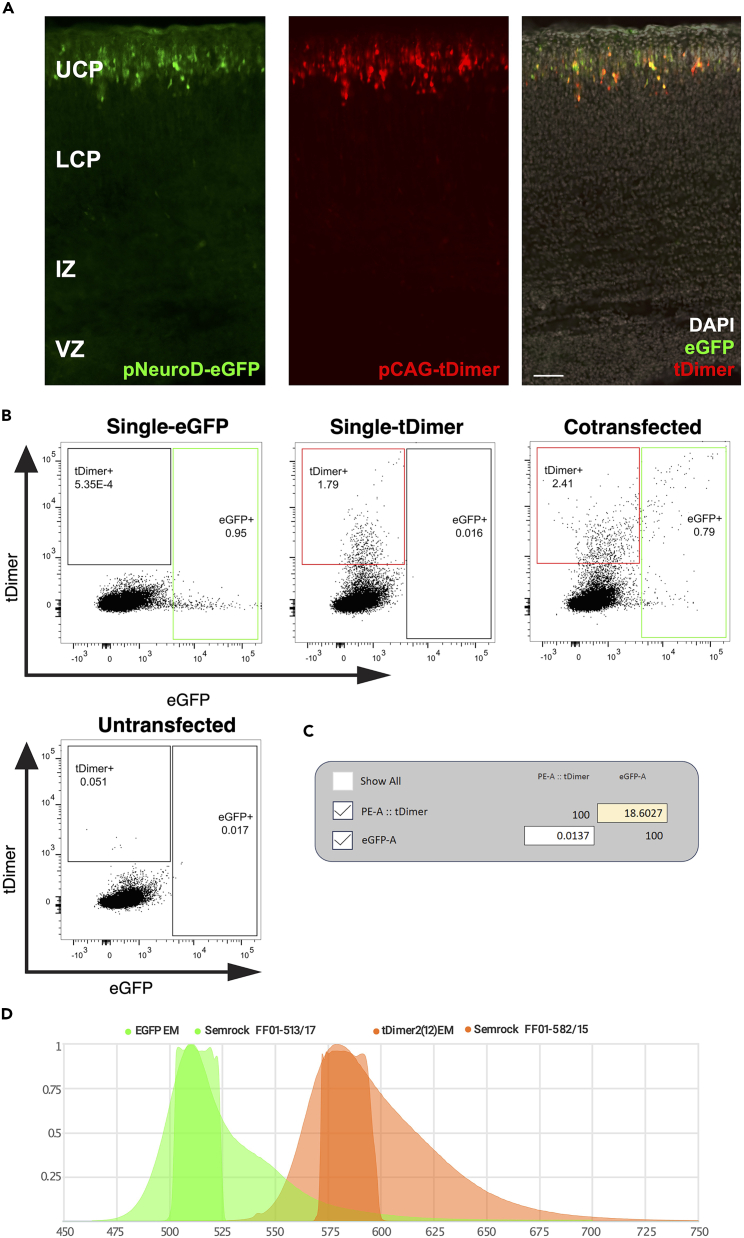
Figure 6.
Gating strategy for separating a cell population into subpopulations by flow cytometry based on the transfection of the construct pNeuroD-eGFP/pCAG-tDimer
(A) Coronal sections of the murine somatosensory cortex of embryonal age E18 after IUE at E14 showing fluorescent protein expression in the target cell population: pNeuroD-eGFP expressed in post-mitotic neurons (left), pCAG-tDimer expressed ubiquitously (middle) and merged (right). UCP: upper cortical plate, LCP: lower cortical plate, IZ: intermediate zone, VZ: ventricular zone. Scale bar: 100 μm.
(B) Singlets SSC populations of both single transfected controls, untransfected, and cotransfected samples with the construct pNeuroD-eGFP/pCAG-tDimer. The FMO control of the single eGFP transfection and the FMO control of the single tDimer transfection, together with the untransfected control, were used to calculate the compensation. Compensation with the FMO signals brighter than the signal in the cotransfected sample ensured correct differential fluorescence detection in the cotransfected sample. This compensation and gate setting served as template every time the cotransfected samples were sorted. Note: Based on our experimental question, eGFP+ cells may also have tDimer fluorescence (double positive). This experiment was performed on 20,000 cells of E18 embryonic cortices.
(C) Computational compensation matrix (“Compensation Wizard” of BD FACS Diva software; recreated with FlowJo) defined values for compensation of spillover into each other BP filter.
(D) The BP filters 582/15 and 513/17 covered well the peaks of the emission spectra of the fluorescent proteins used here, tDimer and eGFP, respectively. The plot was generated with FPbase.17